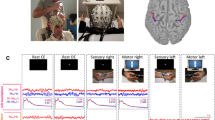
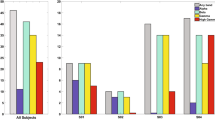
Summary
In awake monkeys, electrical pulse stimuli which mimic touch stimulation were delivered to the thenar eminence while electrophysiologic recordings were made from surface to depth in postcentral gyrus. Cortical depth profiles of somatosensory evoked responses (SEPs), multiple unit activity (MUA) and current source-densities (CSDs) were analyzed to gain insight into the neural process underlying the SEP and somatic sensation. The following was found: 1.) The thenar stimulus evoked four main SEP components which were seen over wide regions of postcentral gyrus: P1 at 12 ms, the primary evoked response; P1a, near 20 ms; N1, near 50 ms; and P2, around 120 ms after the stimulus. 2.) MUA was observed during the P1, P1a and N1 temporal intervals whose vigor changed as a function of the respective SEP component's amplitude. 3.) CSD analysis showed that during P1 a current sink and source appeared within the the middle and superficial cortical layers, respectively. During P1a, a sink just above that of P1 and a superficial source became evident. During N1, a large superficial sink and one or two deep sources appeared. Evoked MUA during P1 and P1a was most prominent at the level of their current sinks while MUA during N1 appeared at the level of the current sources, in general. 4.) When stimulation was moved from the thenar portion of the hand to a region which most closely matched the receptive field of the cortical recording site, P1 and P1a increased amplitude while N1 both increased in amplitude and decreased in peak latency. Also, MUA activity during the early temporal intervals become more vigorous. These changes were similar to those observed with thenar stimuli as the recording site approached the thenar cortical representation. 5.) Over repeated trials at a single stimulus intensity, the spontaneous changes in SEP amplitude were found to be directly correlated with MUA and CSD measures within the same temporal interval. In contrast, SEP, MUA or CSD measures within the early temporal intervals (i.e., P1 or P1a) were uncorrelated or less frequently, inversely correlated with the same measures in the late temporal interval (i.e. N1). The multiple measures complemented one another which led to a descriptive model of the neural process underlying the evoked cortical response in postcentral gyrus of awake monkey.
Similar content being viewed by others
References
Arezzo JC, Vaughan HG Jr, Legatt AD (1981) Topography and intracranial sources of somatosensory evoked potentials in the monkey. II. Cortical components. Electroenceph Clin Neurophysiol 28: 1–18
Barna JS, Arezzo JC, Vaughn HG Jr (1981) A new multielectrode array for the simultaneous recording of field potentials and unit activity. Electroenceph Clin Neurophysiol 52: 494–496
Carlson KR (1972) A temporary restraint chair for monkeys. Physiol Behav 9: 493–494
Cauller LG, Mayhew W, Teyler TJ (1983a) LabMan program and description available from Central Nervous Systems, 5853 Roc Marie Dr., Kent, OH 44240
Cauller LJ, Mayhew W, Teyler TJ (1983b) Discrete sampling of continous biological signals: analog-to-digital conversion. Brain Res Bull 11: 755–760
Cauller LJ, Kulics AT (1984) Depth profiles of somatosensory-evoked potentials, MUA and CSD in somatosensory cortex of awake rhesus monkeys. Abstr Soc Neurosci 10: 492
Corkin S, Milner B, Rasmussen T (1970) Somatosensory thresholds. Contrasting effects of postcentral gyrus and posterior parietal lobe excisions. Arch Neurol 23: 41–58
Eccles JC (1951) Interpretation of action potentials evoked in cerebral cortex. EEG Clin Neurophysiol 3: 449–464
Eccles JC, (1981) The modular operation of the cerebral neocortex considered as the material basis of mental events. Neuroscience 6(10): 1839–1856
Eccles JC (1984) The cerebral neocortex: a theory of its operation. In: Jones E, Peters A (eds) Cerebral cortex: functional properties of cortical cells, Vol 2. Plenum, New York London pp 1–36
Feldman ML (1984) Morphology of the neocortical pyramidal neuron. In: Peters A, Jones EG (eds) Cerebral cortex: cellular components of the cerebral cortex, Vol 1. Plenum, New York London pp 123–200
Ferris CD (1974) Introduction to bioelectrodes, Chap 2. Plenum, New York, pp 25–29
Gardner EP, Costanzo RM (1980a) Spatial integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 34(2): 420–443
Gardner EP, Costanzo RM (1980b) Temporal integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 34(2): 444–468
Gardner E, Hämäläinen H, Warren S, Davis J, Young W (1984) Somatosensory evoked potentials (SEPs) and cortical single unit responses elicited by mechanical tactile stimuli in awake monkeys. Electroenceph Clin Neurophysiol 58: 537–552
Gibson R (1968) Electrical stimulation of pain and touch. In: Kenshalo D (ed) The skin senses. Charles C Thomas, Springfield IL, pp 223–261
Hyvärinen J (1982) The parietal cortex of monkey and man. In: Braitenberg V (ed) Studies of brain function, Vol 8. Springer, New York, 202 pp
Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O (1983) Converging patterns of finger representation and complex response properties of neurons in area 1 of the first somatosensory cortex of the conscious monkey. Exp Brain Res 51: 327–337
Jones EG (1984) Laminar distribution of cortical efferent cells. In: Peters A, Jones EG (eds) Cerebral cortex: cellular components of the cerebral cortex, Vol 1. Plenum, New York London pp 521–553
Kaas JH (1983) What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63: 206–230
Kulics AT (1977) Sensory discriminability comparisons in human and monkey with implications for the study of central nervous correlates. Ann NY Acad Sci 299: 244–254
Kulics AT (1982) Cortical neural evoked correlates of somatosensory stimulus detection in the rhesus monkey. Electroenceph Clin Neurophysiol 53: 78–93
Kulics AT, Lineberry CG, Roppolo JR (1977) Neurophysiological correlates of cutaneous discrimination performance in rhesus monkey. Brain Res 136: 360–365
Kuperstein M, Whittington DA (1981) A practical 24 channel microelectrode for neural recording in vivo. IEEE Trans Biomed Eng BME-28: 288–293
Laskin SE, Spencer WA (1979) Cutaneous masking. II. Geometry of excitatory and inhibitory receptive fields of single units in somatosensory cortex of the cat. J Neurophysiol 42: 1061–1082
Libet B, Alberts WW, Wright EW, Feinstein B (1967) Responses of human somatosensory cortex to stimuli below threshold for conscious sensation. Science 158: 1597–1600
Lineberry CG, Kulics AT (1978) The effects of diazepam, morphine and lidocaine on nociception in rhesus monkeys: a signal detection analysis. J Pharmacol Exp Ther 205: 302–310
McKenna T, Whitsel B, Dreyer D (1982) Anterior parietal cortical topographic organization in Macaque monkey: a reevaluation. J Neurophysiol 48: 289–317
Mitzdorf U (1985) Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65(1): 37–100
Mountcastle VB, Talbot WH, Sakata H, Hyvärinen J (1969) Cortical neural mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32: 452–484
Nelson RJ, Sur M, Felleman D, Kaas J (1980) Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol 192: 611–643
Nicholson C (1979) Generation and analysis of extracellular field potentials. In: Electrophysiological techniques. Society for Neuroscience Short Course, 93–147
Norrsell U (1980) Behavioral studies of the somatosensory system. Physiol Rev 60: 327–354
Penfield W, Rasmussen T (1950) The cerebral cortex of man. A clinical study of localization of function. Macmillan, New York, pp 248
Prohaska O, Pacha F, Pfundner P, Petsche H (1979) A 16-fold semi-microelectrode for intracortical recording of field potentials. Electroenceph Clin Neurophysiol 47: 629–631
Rappelsberger P, Pockberger H, Petsche H (1981) Current source density analysis: methods and application to simultaneously recorded field potentials of the rabbit's visual cortex. Pflügers Arch 389: 159–170
Semmes J (1973) Somesthetic effects of damage to the central nervous system. In: A Iggo (ed) Somatosensory system, Vol 2. Springer, New York, pp 719–742
Semmes J, Weinstein S, Ghent L, Teuber HL (1960) Somatosensory changes after penetrating brain wounds in man. Harvard University Press, Cambridge, 91 pp
Szentagothai J (1975) The module-concept in cerebral cortex architecture. Brain Res 95: 475–496
Szentagothai J (1978) The Ferner lecture, 1977. The neuron network of the cerebral cortex: a functional interpretation. Proc R Soc Lond B 201: 219–248
Uttal WR (1973) The psychobiology of sensory coding. Harper and Row, New York, 663 pp
Vallbo AB, Olsson KA, Westberg KG, Clark FJ (1984) Microstimulation of single tactile afferents from the human hand. Sensory attributes related to unit type and properties of receptive fields. Brain 107: 727–749
Vaughan HG, Jr, (1982) The neural origins of human event-related potentials. Ann NY Acad Sci 388: 125–138
Wiesenfeld-Hallin Z, Hallin RG, Persson A (1984) Do large diameter cutaneous afferents have a role in the transmission of nociceptive messages? Brain Res 311: 375–379
Woolsey CN, Erickson TC (1950) Study of the postcentral gyrus of man by the evoked potential technique. Trans Am Neurol Assoc 75: 50–52
Woolsey CN, Marshall LL, Bard WH (1942) Representation of cutaneous tactile sensibility in the cerebral cortex of the monkey as indicated by evoked potentials. Bull John Hopkins Hosp 70: 399
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kulics, A.T., Cauller, L.J. Cerebral cortical somatosensory evoked responses, multiple unit activity and current source-densities: their interrelationships and significance to somatic sensation as revealed by stimulation of the awake monkey's hand. Exp Brain Res 62, 46–60 (1986). https://doi.org/10.1007/BF00237402
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00237402