Summary
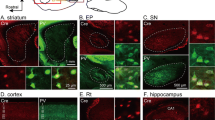
Unilateral injection of Fast Blue retrograde fluorescent neuron tracer into the parolfactory lobe (POL) in the quail showed multiple innervation of this structure. Neurons projecting into the POL were located in three areas: 1) the telencephalon, where they were scattered in the paleostriatum, the archistriatum and ventral hyperstriatum, and among the fibers of different tracts including the anterior commissure, the occipito-mesencephalic tract and the fasciculus prosencephali lateralis; 2) the diencephalon, where fluorescent neurons with large multipolar perikarya were found in the dorsal thalamic wall; 3) the midbrain, where large perikarya were located in the ventralis area of Tsai, the locus coeruleus, the nucleus subcoeruleus, around the medial longitudinal fasciculus, in the substantia grisea centralis, the formatio reticularis mesencephali and among the fibers of the brachium conjunctivum. In most cases, axons innervating the POL ran parallel to the fibers of the medial forebrain bundle and contralateral to the perikarya from which they originated. This study also showed that the anterior and posterior parts of the POL received fibres from different sources.
Similar content being viewed by others
References
Ariëns Kappers CU, Huber GC, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates, including man, 2 Vols. Macmillan, New York (Reprinted in 1960 by Hafner, New York in 3 Vols)
Baylé JD, Ramade F, Oliver J (1974) Stereotaxic topography of the brain of the quail, Coturnix coturnix japonica. J Physiol (Paris) 68: 219–241
Benoît J (1964) The role of the eye and of the hypothalamus in the photostimulation of gonads in the duck. Ann NY Acad Sci 117: 204–217
Benoît J (1970) Etude de l'action des radiations visibles sur la gonadostimulation et leur pénétration ultra-crânienne chez les oiseaux et les mammifères. Benoît J, Assenmacher I (eds) CNRS, Paris 121–149
Benoît J, Kehl R (1939) Nouvelles recherches sur les voies nerveuses photoréceptrices et hypophysostimulantes chez le Canard domestique. CR Soc Biol (Paris) 131: 89–93
Benowitz LI, Karten HJ (1976) The tractus infundibuli and other afferents to the parahippocampal region of the pigeon. Brain Res 102: 174–180
Bentivoglio M, Kuypers HGJM, Catsman-Berrevoets CE, Dann O (1979) Fluorescent retrograde neuronal labeling in rat by means of substances binding specifically to adenine-thymine rich DNA. Neurosci Lett 12: 235–240
Bentivoglio M, Kuypers HGJM, Catsman-Berrevoets CE, Loewe H, Dann O (1980) Two new fluorescent retrograde neuronal tracers which are transported over long distances. Neurosci Lett 18: 25–30
Bertler A, Falk B, Gottfried CG, Ljunggren L, Rosengren E (1964) Some observations on adrenergic connections between mesencephalon and cerebral hemispheres. Acta Pharmacol 21: 283–289
Bons N (1976) Retino-hypothalamic pathway in the duck, Anas platyrhynchos. Cell Tiss Res 168: 343–360
Bons N, Jallageas M, Alonso G (1984) Hypothalamic neurohormones in birds: LHRH, vasotocin and mesotocin systems. Abstr 3rd Int Symp on Avian Endocrinology, New Brunswick-New Jersey 25–26 juin 1984
Bons N, Jallageas M, Assenmacher I (1975) Influence des photorécepteurs rétiniens et extrarétiniens dans la stimulation testiculaire de la caille par des jours longs. J Physiol (Paris) 71: 265–266 A
Bons N, Kerdelhué B, Assenmacher I (1978) Mise en évidence d'un deuxième système neurosécrétoire à LH-RH dans l'hypothalamus du canard. CR Acad Sci (Paris) 287: 145–148
Bons N, Oliver J (1984) Afférences aux lobes paraolfactifs chez la caille: étude à l'aide d'un traceur fluorescent. Ann d'Endocr (Paris) 45: 16N
Cooper ML, Pickard GE, Silver R (1983) Retinohypothalamic pathway in the dove demonstrated by anterograde HRP. Brain Res Bull 10: 715–718
Davies DT (1980) The neuroendocrine control of gonadotrophin release in the Japanese quail. III. The role of the tuberal and anterior hypothalamus in the control of ovarian development and ovulation. Proc R Soc (Lond) 206: 421–437
Davies DT, Follet BK (1980) Neuroendocrine regulation of gonadotrophin-releasing hormone secretion in the Japanese quail. Gen Comp Endocr 40: 220–225
Foster RG, Follet BK, Lythgoe JN (1985) Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature 313: 50–52
Glass JD, Lauber JK (1981) Sites and action spectra for encephalic photoreception in the Japanese quail. Am J Physiol 240: 220–228
Gwinner E, Turek FW, Smith SO (1971) Extraocular light perception in photoperiodic responses of the white-crowned sparrow (Zonotrichia leucophrys) and of golden-crowned sparrow (Z. atricapllla). Z Vergl Physiol 75: 323–331
Harrison PC (1972) Extraretinal photocontrol of reproductive responses of leghorn hens to photoperiods of different length and spectrum. Poultry Sci 51: 2060–2064
Hartwig HG (1974) Electron microscopic evidence for a retinohypothalamic projection to the suprachiasmatic nucleus of Passer domesticus. Cell Tiss Res 147: 491–504
Homma K, Sakakibara Y (1971) Encephalic photoreceptors and their significance in photoperiodic control of sexual activity in Japanese quail. In: Menaker M (ed) Biochronometry. Natl Acad Sci, Washington, pp 333–341
Homma K, Sakakibara Y, Ohta M (1977) Potential sites and action spectra for encephalic photoreception in the Japanese quail. In: Follett BK (ed) First Internat Symp Avian Endocrinology, Calcutta. Abstr 25–26
Homma K, Wilson WO, Siopes TD (1972) Eyes have a role in photoperiodic control of sexual activity of Coturnix. Science 178: 421–423
Illing RB (1980) Axonal bifurcation of cat retinal ganglion cells as demonstrated by retrograde double labeling with fluorescent dyes. Neurosci Lett 19: 125–130
Karten HJ, Dubbeldam JL (1973) The organization and projections of the palaeostriatal complex in the pigeon (Columbia livia) J Comp Neurol 148: 61–90
Jallageas M, Bons N, Daniel JY, Assenmacher I (1978) The endocrine control of the reproductive cycle in male ducks. Pavo 16: 67–88
Karten HJ, Hodos W (1967) A Stereotaxic atlas of the brain of the pigeon (Columbia livia). The Johns Hopkins Press, Baltimore
Kato M, Kato Y, Oishi T (1967) Radioluminous paints as activator of photoreceptor system studied with swallow-tail butterfly and quail. Proc Jpn Acad 43: 220–223
Kuypers HGJM, Catsman-Berrevoets CE, Padt RE (1977) Retrograde axonal transport of fluorescent substances in the rat's brain. Neurosci Lett 6: 127–135
McMillan JP, Underwood HA, Elliott JA, Stetson MH, Menaker M (1975) Extraretical light photoreception in the sparrow. IV. Further evidence that the eyes do not participate in photoperiodic photoreception. J Comp Physiol 97: 205–213
McNeill TH, Kozlowski GP, Abel JH Jr, Zimmerman EA (1976) Neurosecretory pathways in the mallard duck (Anas platyrhynchos) brain: localization by aldehyde fuchsin and immuniperoxidase techniques for neurophysin NP and gonadotropin releasing hormone (Gn-RH). Endocrinology 99: 1323–1332
Menaker M (1971) Rhythms, reproduction, and photoreception. Biol Reprod 4: 295–308
Menaker M, Roberts R, Eliott J, Underwood H (1970) Extraretinal light perception in the sparrow. III. The eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci USA 67: 320–325
Menaker M, Underwood H (1976) Extraretinal photoreception in birds. Photochem Photobiol 23: 299–306
Nottebohm F, Stokes TM, Leonard CM (1976) Central control of song in the canary, Serinus canarius. J Comp Neurol 165: 457–486
Oishi T, Lauber JK (1973) Photoreception in the photosexual response of quail. I. Site of photoreceptor. Am J Physiol 225: 155–158. II. Effects of intensity and wavelength. Am J Physiol 225: 880–886
Oliver J, Baylé JD (1982) Brain photoreceptors for the photo-induced testicular response in birds. Experientia 38: 1021–1029
Oliver J, Bouillé C, Herbuté S, Baylé JD (1977) Horseradish peroxidase study of intact or deafferented infundibular complex in Coturnix quail. Neuroscience 2: 989–996
Ookawa T (1970) Effets of bilateral optic enucleation on body growth and gonads in young male chicks. Poultry Sci 49: 333–334
Shimizu I, Yoshimoto M, Kojima T, Okado N (1984) Development of retinohypothalamic projections in the chick embryo. Neurosci Lett 50: 43–47
Sicard B (1982) La photo-gonado-stimulation chez la Caille. Etude de la photoréactivité cérébrale. Thèse Montpellier, pp 103
Spatz WB, Grabig S (1983) Reduced fading of fast blue in the brain of the guinea-pig by treatment with sodium-nitroprusside. Neurosci Lett 38: 1–4
Sterling RJ, Sharp PJ (1982) The localization of LHRH neurones in the diencephalon of the domestic hen. Cell Tiss Res 222: 283–298
Stetson MH (1972) Hypothalamic regulation of testicular function in Japanese quail. Z Zellforsch 130: 389–410
Turek FK (1975) Extraretinal photoreception during the gonadal photoreceptory period in the golden-crowned sparrow. J Comp Physiol 96: 27–36
Underwood H (1975) Retinally perceived photoperiod does not influence subsequent testicular regression in house sparrows. Gen Comp Endocr 27: 475–478
Underwood H, Menaker M (1970) Photoreception in sparrows. Science 172: 293
Wada M (1974) Blockade of photoperiodically-induced testicular growth by hypothalamic deafferentation in Japanese quail (Coturnix coturnix japonica). Gen Comp Endocr 24: 113–120
Wilson FE (1967) The tubero-infundibular neurosystem: a component of the photoperiodic control mechanism of the whitecrowned sparrow, Zonotrichia leucophrys gambelii. Z Zellforsch 82: 1–24
Yokoyama K, Oksche A, Darden JR, Farner DS (1978) The sites of encephalic photoreception in photoperiodic induction of the growth of the testes in the white-crowned sparrow (Zonotrichia leucophrys gambelii). Cell Tiss Res 189: 441–467
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bons, N., Oliver, J. Origin of the afferent connections to the parolfactory lobe in quail shown by retrograde labelling with a fluorescent neuron tracer. Exp Brain Res 63, 125–134 (1986). https://doi.org/10.1007/BF00235654
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235654