Summary
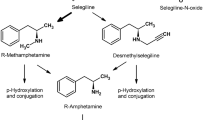
Selegiline (10 mg per day) selectively inhibits monoamine oxidase type B and thus thwarts the metabolism of dopamine by this enzyme. Selegiline has been used in the therapy of Parkinson's disease since 1986. It enhances the efficacy of levodopa, allows a reduction of the levodopa dose, and improves fluctuations in disability. It also interacts with mechanisms suspected of playing a role in the progression of the disease. Animal studies have shown that selegiline prevents the development of a Parkinson-like syndrome induced by the neurotoxin MPTP. It decreases oxidative stress resulting from the metabolism of dopamine via MAO-B. Clinical studies have shown that selegiline is effective in the therapy of untreated de novo patients the progression of symptoms demanding the introduction of levodopa into the therapy was delayed, and the risk of needing levodopa treatment within one year was reduced by 57% with selegiline. The mode of action of this drug in the treatment of early Parkinson's disease is still under discussion. There is strong evidence that selegiline may slow the progression of the disease, but a direct symptomatic effect cannot be excluded.
Similar content being viewed by others
Abbreviations
- MAO-B:
-
monoamine oxidase-B
- MAO-BI:
-
MAO-B inhibitor
- MPTP:
-
1-methyl-4-phenyl-1,2.3,6-tetrahydropyridine
References
Birkmayer W, Hornykiewicz O (1966) The levodopa effect in Parkinson's syndrome in man. Arch Psychiatr Nervenkr 203:560–574
Birkmayer W, Riederer P, Youdim MBH, Linauer W (1975) The potentiation of the anti a kinetic effect after l-dopa treatment by an inhibitor of MAO-B, Deprenil. J Neural Transm 36:303–326
Birkmayer W, Knoll J, Riederer P, Youdim MBH, Hars V, Marton J (1985) Increased life expectancy resulting from addition of l-deprenyl to madopar. Treatment in Parkinson's disease: a long-term study. J Neural Transm 64:113–127
Cohen G, Spina MB (1989) Deprenyl suppresses the oxidant stress associated with increased dopamine turnover. Ann Neurol 26:689–690
Carrillo MC, Kanai S, Nokubo M, Kitani K (1991) (−)Deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Science 48:517–521
Finnegan KT, Skratt JJ, Irwin I, DeLanney LE, Langston JW (1990) Protection against DSP-4-induced neurotoxicity by deprenyl is not related to its inhibition of MAO B. Fur J Pharmacol 184:119–126
Knoll J (1978) The possible mechanism of action of (−)-deprenyl in Parkinson's disease. J Neural Transm 43:177–198
Knoll J (1988) Extension of life span of rats by long-term (−)-deprenyl treatment. Mount Sinai J Med 55:67–74
Myllylä VV, Sotaniemi KA, Tuominen J, Heinonen E (1989) Selegiline as primary treatment in early phase Parkinson's disease — an interim report. Acta Neurol Scand 126:177–182
The Parkinson Study Group (1989) Effect of deprenyl on the progression of disability in early Parkinson's disease (DATATOP). N Engl J Med 16:1364–1371
Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MBH (1989) Transition metals, ferritin, glutathione and ascorbic acid in Parkinsonian brains. J Neurochem 52:515–520
Rinne JO, Röyttä M, Paljärvi L, Rummukainen J, Rinne UK (1991) Selegiline (deprenyl) treatment and death of nigral neurons in Parkinson's disease. Neurology 41:859–861
Tetrud JW, Langston JW (1989) The effect of deprenyl (selegiline) on the natural history of Parkinson's disease. Science 245:519–522
Youdim MBH, Ben-Shachar D, Riederer P (1989) Is Parkinson's disease a progressive siderosis of substantia nigra resulting in iron and melanin neurodegeneration? Acta Neurol Scand 126:47–54
Josephson MA, Singh BN (1988) Hemodynamic effects of class III antiarrhythmic agents. In: Singh BN (ed) Control of cardiac arrhythmias by lengthening repolarization. Futura, New York, pp 153–160
Mostow ND, Vrobel TR, Noon D, et al (1986) Rapid suppression of complex ventricular arrhythmias with high-dose oral amiodarone. Circulation 73:1231–1238
Mukharji J, Rude RE, Poole WK, et al (1984) Risk factors for sudden death after acute myocardial infarction: two year follow-up. Am J Cardiol 54:31–36
Nademanee K, Singh BN, Intarachot V, et al (1983) Amiodarone in refractory life-threatening ventricular arrhythmias. Ann Intern Med 98 [Part 1]:577–584
Pfisterer M, Burkart F, Milller-Brand J (1985) Important differences between short- and long-term hemodynamic effects of amiodarone in patients with chronic ischemic heart disease at rest and during ischemia-induced left ventricular dysfunction. J Am Coll Cardiol 5:1205–1209
Podrid PJ, Schoeneberger A, Lown B (1980) Congestive heart failure caused by oral disopyramide. N Engl J Med 302:614–617
Remme WJ, Hoogenhyze DCA van, Krauss XH, et al (1985) Acute hemodynamic and antiischemic effects of intravenous amiodarone. Am J Cardiol 55:639–644
Scheininger M, Silber S, Theisen F, et al (1986) Einfluß von Amiodaron auf die linksventrikuläre Auswurffraktion bei Patienten mit eingeschränkter Auswurffraktion und komplexen Rhythmusstörungen. Intensivmed Prax 23:74–78
Singh BN, Jenitt DE, Downey JM (1976) Effects of amiodarone and L 8040, novel antianginal and antiarrhythmic drugs, on cardiac and coronary hemodynamics and on cardiac intracellular potentials. Clin Exp Pharmacol Physiol 3:427–431
Sugrue DD, Dickie S, Myers MJ, et al (1984) Effect of amiodarone on left ventricular ejection and filling in hypertrophic cardiomyopathy as assessed by radionuclide angiography. Am J Cardiol 54:1054–1058
Swerdlow CD, Winkle RA, Mason JW (1983) Determinations of survival in patients with ventricular tachyarrhythmias. N Engl J Med 308:1436–1440
Trobaugh GB, Kudenchuk PJ, Greene HL, et al (1984) Effect of amiodarone on ventricular function as measured by gated radionuclide angiography. Am J Cardiol 54:1263–1266
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wessel, K., Szelenyi, I. Selegiline — An overview of its role in the treatment of Parkinson's disease. Clin Investig 70, 459–462 (1992). https://doi.org/10.1007/BF00235534
Issue Date:
DOI: https://doi.org/10.1007/BF00235534