Abstract
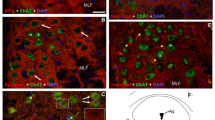
Embryonic cholinesterases are assigned important functions during morphogenesis. Here we describe the expression of butyrylcholinesterase and acetylcholinesterase, and the binding of peanut agglutinin, and relate the results to mitotic activity in chick wing and leg buds from embryonic day 4 to embryonic day 9. During early stages, butyrylcholinesterase is elevated in cells under the apical ectodermal ridge and around invading motoraxons, while acetylcholinesterase is found in the chondrogenic core, on motoraxons and along the ectoderm. Peanut agglutinin binds to the apical ectodermal ridge and most prominently to the chondrogenic core. Measurements of thymidine incorporation and enzyme activities were consistent with our histological findings. Butyrylcholinesterase is concentrated near proliferative zones and periods, while acetylcholinesterase is associated with low proliferative activity. At late stages of limb development, acetylcholinesterase is concentrated in muscles and nonexistent within bones, while butyrylcholinesterase shows an inverse pattern. Thus, as in other systems, in limb formation butyrylcholinesterase is a transmitotic marker preceding differentiation, acetylcholinesterase is found on navigating axons, while peanut agglutinin appears in non-invaded regions. These data suggest roles for cholinesterases as positive regulators and peanut-agglutinin-binding proteins as negative regulators of neural differentiation.
Similar content being viewed by others
References
Barthalay Y, Hipeau-Jacquotte R, De la Escalera S, Jimenez F, Piovant M (1990) Drosophila neurotactin mediates heterophilic cell adhesion. EMBO J 9:3603–3609
Bottenstein E, Sato GH (1979) Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA 76:514–517
Christie DL, Cleverly DR, O'Connor CJ (1991) Human milk bilesalt stimulated lipase. Sequence similarity with rat lysophospholipase and homology with the active site region of cholinesterases. FEBS Lett 278:190–194
Cooper NGF, Steindler DA (1986) Lectins demarcate the barrel subfield in the somatosensory cortex of the early postnatal mouse, J Comp Neurol 249:157–169
Davies JA, Cook GMW, Stern CD, Keynes RJ (1990) Isolation from chick somites of a glycoprotein fraction that causes collapse of dorsal root ganglion growth cones. Neuron 2:11–20
De la Escalera S, Bockamp EO, Moya F, Piovant M, Jiménez F (1990) Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J 9:3593–3601
Drews U (1975) Cholinesterase in embryonic development. Prog Histochem Cytochem 7:1–53
Drews U, Drews U (1972) Cholinesterase in der Extremitätenentwicklung des Hühnchens. I. Die Phasen der Cholinesterase-Aktivität in der jungen Knospe und bei der Abgrenzung von Knorpel- und Muskelanlagen. Wilhelm Roux' Arch Entwicklungsmech Org 169:70–86
Drews U, Drews U (1973) Cholinesterase in der Extremitätenentwicklung des Hühnchens. II. Fermentaktivität und Bewegungsverhalten der präsumptiven Knorpelzellen in vitro. Wilhelm Roux' Arch Dev Biol 173:208–227
Drews U, Schmidt H, Oettling G, Vanittanakom P (1986) Embryonic Cholinesterase in the chick limb bud. Acta Histochem [Suppl] 32:133–137
Ellman GL, Courtney DK, Anders V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Falugi C, Raineri M (1985) Acetylcholinesterase (AChE) and pseudocholinesterase (BuChE) activity distribution pattern in early developing chick limbs. J Embryol Exp Morphol 86:89–108
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hawkes R, Niday E, Gordon J (1982) A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem 119:142–147
Herrup K (1987) Glial cells and the formation of invisible boundaries in development (or, peanut barrels in the brain). Trends Neurosci 10:443–444
Illing RB, Graybiel AM (1985) Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cats superior colliculus. Neuroscience 14:455–482
Karnovsky MJ, Roots LJ (1964) A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem 12:219–221
Korr H (1981) Techniques in neuroanatomical research. Springer, Berlin Heidelberg New York, pp 218–244
Krejci E, Duval N, Chatonnet A, Vincens P, Massoulié J (1991) Cholinesterase-like domains in enzymes and structural proteins: functional and evolutionary relationships and identification of a catalytically essential aspartic acid. Proc Natl Acad Sci USA 88:6647–6651
Kristt DA (1989) Acetylcholinesterase in immature thalamic neurons: relation to afferentation, development, regulation and cellular distribution. Neuroscience 29:27–43
Layer PG (1983) Comparative localization of acetylcholinesterase and pseudocholinesterase during morphogenesis of the chick brain. Proc Natl Acad Sci USA 80:6413–6417
Layer PG (1990) Cholinesterases preceding major tracts in vertebrate neurogenesis. Bioessays 12:415–420
Layer PG, Alber R (1990) Patterning of early chick brain vesicles as revealed by peanut agglutinin and cholinesterases. Development 109:613–624
Layer PG, Kaulich S (1991) Cranial nerve growth in birds is preceded by cholinesterase expression during neural crest cell migration and the formation of an HNK-1 scaffold. Cell Tissue Res 265:393–407
Layer PG, Sporns O (1987) Spatiotemporal relationship of embryonic cholinesterases with cell proliferation in chicken brain and eye. Proc Natl Acad Sci USA 84:284–288
Layer PG, Willbold E (1993) Cholinesterases in avian neurogenesis. Int Rev Cytol 151:139–181
Layer PG, Alber R, Sporns O (1987) Quantitative development and molecular forms of acetyl- and butyrylcholinesterase during morphogenesis and synaptogenesis of chick brain and retina. J Neurochem 49:175–182
Layer PG, Alber R, Rathjen FG (1988a) Sequential activation of butyrylcholinesterase in rostral half somites and acetyl-cholinesterase in motoneurones and myotomes preceding growth of motor axons. Development 102:387–396
Layer PG, Rommel S, Bülthoff H, Hengstenberg R (1988b) Independent spatial waves of biochemical differentiation along the surface of chicken brain as revealed by the sequential expression of acetylcholinesterase. Cell Tissue Res 251:587–595
Liu L, Layer PG (1984) Spatio-temporal patterns of differentiation of whole heads of the embryonic chick as revealed by binding of a FITC-coupled peanut-agglutinin (FITC-PNA). Dev Brain Res 12:173–182
Liu L, Layer PG (1988) Peanut-agglutinin receptors during development of the chick visual system: a structural, functional and molecular analysis. In: Yew DT, So KF, Tsang DSC (eds) Vision: structure and function. World Scientific, Singapore, pp 550–580
Lowry DH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM (1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41:31–91
Miki A, Mizoguti H (1982a) Proliferating ability, morphological development and acetylcholinesterase activity of the neural tube cells in early chick embryos. An electron microscopic study. Histochemistry 76:303–314
Miki A, Mizoguti H (1982b) Acetylcholinesterase activity in the myotome of the early chick embryo. Cell Tissue Res 227:23–40
Mizoguti H, Miki A (1985) Interrelationship among the proliferating ability, morphological development and acetylcholinesterase activity of the neural tube cells in early chick embryos. Acta Histochem Cytochem 18:85–96
Olson PF, Fessier LI, Nelson RE, Stern RE, Campbell AG, Fessler JH (1990) Glutactin, a novel Drosophila basement membrane-related glycoprotein with sequence similarity to serine esterases. EMBO J 9:1219–1227
Puelles L, Amat JA, Martinez-de-la-Torre M (1987) Segment-related, mosaic neurogenetic pattern in the forebrain and mesencephalon of early chicken embryos. 1. Topography of AChE-positive neuroblasts up to stage HH 18. J Comp Neurol 266:247–268
Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U (1987) Membrane glycoproteins involved in neurite fasciculation. J Cell Biol 104:343–353
Richards GM (1974) Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem 57:369–376
Robertson RT, Hanes MA, Yu J (1988) Investigations of the origins of transient acetylcholinesterase activity in developing rat visual cortex. Dev Brain Res 41:1–23
Robertson RT, Yu J (1993) Acetylcholinesterase and neural development — new tricks for an old dog. News Physiol Sci 8:266–272
Schmidt H (1981) Muscarinic acetylcholine receptor in the chick limb bud during morphogenesis. Histochemistry 71:89–98
Schröder C (1980) Characterization of embryonic cholinesterase in chick limb bud by colorimetry and disk electrophoresis. Histochemistry 69:243–253
Schumacher M, Camp S, Maulet Y, Newton M, MacPhee-Quigley K, Taylor SS, Friedman T, Taylor P (1986) Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. Nature 319:407–409
Schwab ME, Kapfhammer JP, Bandtlow CE (1993) Inhibitors of neurite growth. Annu Rev Neurosci 16:565–595
Sethi JS, Tewari HB (1976) Histoenzymological mapping of acetyl-cholinesterase and butyrylcholinesterase in the diencephalon and mesencephalon of Uromastix hardwickii. J Hirnforsch 17:335–349
Silver A (1974) The biology of cholinesterases. North-Holland, Amsterdam
Slack JMW (1985) Peanut lectin receptors in the early amphibian embryo: regional markers for the study of embryonic induction. Cell 41:237–247
Soreq H, Zakut H (1993) Human cholinesterases and anti-cholinesterases. Academic Press, San Diego London
Steindler DA, Cooper NGF (1987) Glial and glycoconjugate boundaries during postnatal development of the central nervous system. Dev Brain Res 36:27–38
Stern CD, Sisodiya SM, Keynes RJ (1986) Interaction between neurites and somite cells: inhibition and stimulation of nerve growth in the chick embryo. J Embryol Exp Morphol 91:209–226
Tosney KW, Landmesser LT (1985) Development of the major pathways for neurite outgrowth in the chick hindlimb. Dev Biol 109:193–214
Tosney KW, Oakley A (1990) The perinotochordal mesenchyme acts as a barrier to axon advance in the chick embryo: implications for a general mechanism of axonal guidance. Exp Neurol 109:75–89
Vanittanakom P, Drews U (1985) Ultrastructural localization of cholinesterase during chondrogenesis and myogenesis in the chick limb bud. Anat Embryol 172:183–194
Vollmer G, Layer PG (1986) An in vitro model of proliferation and differentiation of the chick retina: coaggregates of retinal and pigment epithelial cells. J Neurosci 6:1885–1896
Weikert T (1993) Butyrylcholinesterasen und Peanut Agglutinin bindende Proteine als positive und negative Regulatoren des Neuritenwachstums im Hühnerembryo. Immunologische, biochemische und histochemische Untersuchungen. Dissertation, University of Tübingen, Germany
Weikert T, Rathjen FG, Layer PG (1990) Developmental maps of acetylcholinesterase and G4-antigen of the early chicken brain: long-distance tracts originate from AChE-producing cell bodies. J Neurobiol 21:482–498
Weikert T, Ebert C, Rasched I, Layer PG (1994) Novel inactive and distinctively glycosylated forms of butyrylcholinesterase from chicken serum. J Neurochem 63:318–325
Willbold E, Layer PG (1992) A hidden retinal regenerative capacity from the chick ciliary margin is reactivated in vitro, that is accompanied by down-regulation of butyrylcholinesterase. Bur J Neurosci 4:210–220
Wilson SW, Placzek M, Furley AJ (1993) Border disputes: do boundaries play a role in growth-cone guidance? Trends Neurosci 16:316–323
Wolfgang WJ, Forte MA (1989) Expression of acetylcholinesterase during visual system development in Drosophila. Dev Biol 131:321–330
Zacks SI (1952) Esterases in the early chick embryo. Anat Rec 112:509–537
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Alber, R., Sporns, O., Weikert, T. et al. Cholinesterases and peanut agglutinin binding related to cell proliferation and axonal growth in embryonic chick limbs. Anat Embryol 190, 429–438 (1994). https://doi.org/10.1007/BF00235489
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235489