Abstract
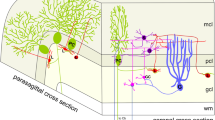
The cerebellum is organized into a series of parasagittally aligned bands that may be revealed histologically in the adult mouse by largely complementary immunostaining of Purkinje cell sets with the monoclonal antibodies Zebrin II (ZII; antigen:aldolase C) and P-path (PP; antigen:90-acetyl glycolipids). We compared the normal staining pattern using these markers and an antibody to calbindin with that found in the reeler mutants (rl/rl), in which most Purkinje cell migration is halted beneath the cerebellar white matter. The results revealed that Purkinje cells in reeler mutants, despite their ectopic location in large subcortical masses, show a clear tendency to distribute into alternating zones that either stain for Zebrin II or for P-path, with variable transition zones of mixed labeling. However, the estimated number of zones was fewer than in the normal adult cortex: roughly 7–9 zones are revealed per side in the mutant compared with 14 major divisions in wild type mice. These results raise the possibility that neurons destined to express these markers are segregated during their migration and that the final phase of migration into the cortex might involve further splitting or interdigitation between cell sets expressing the two antigens.
Similar content being viewed by others
References
Arsenio-Numes, Sotelo C (1985) Development of the spinocerebellar system in the postnatal rat. J Comp Neurol 237:291–306
Blatt GJ, Eisenman LM (1988) Topographic and zonal organization of the olivocerebellar projection in the reeler mutant mouse. J Comp Neurol 267:603–615
Boegman RJ, Parent A, Hawkes R (1988) Zonation in the rat cerebellar cortex: patches of high acetyl-cholinesterase activity in the granular layer are congruent with Purkinje cell compartments. Brain Res 448:237–251
Brochu G, Maler L, Hawkes R (1990) Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol 291:538–552
Buisseret-Delmas C, Angaut P (1993) The cerebellar olivo-corticonuclear connections in the rat. Prog Neurobiol 40:63–87
Caviness VS Jr, Crandall JE, Edwards MA (1988) The reeler malformation: implications for neocortical histogenesis. In: Peters A, Jones EG (eds) Cerebral cortex vol 7. Plenum Press, New York, pp 159–89
Chedotal A, Sotelo C (1992) Early development of olivocerebellar projections in the fetal rat using CGRP immunohistochemistry. Eur J Neurosci 4:1159–1179
Chou DKH, Flores S, Jungalwala FB (1990) Identification of disialosyl paragloboside and O-acetyldisialosyl paragloboside in cerebellum and embryonic cerebrum. J Neurochem 54:1598–1607
Edwards MA, Crandall JE, Leclerc N, Yamamoto M (1994) Effects of nervous mutation on Purkinje cell compartments defined by Zebrin II and 90-acetylated gangliosides expression. Neurosci Res 19:167–174
Eisenman LM (1988) Histochemical localization of 5′-nucleotidase in the reeler mutant mouse. Neurosci Lett 94:70–75
Eisenman LM, Hawkes R (1989) 5′-Nucleotidase and the mabQ113 antigen share a common distribution in the cerebellar cortex of the mouse. Neuroscience 31:231–235
Eisenman LM, Hawkes R (1993) Antigenic compartmentation in the mouse cerebellar cortex: Zebrin and HNK-1 reveal a complex, overlapping molecular topography. J Comp Neurol 335:586–605
Goffinet AM (1983) The embryonic development of the cerebellum in normal and reeler mutant mice. Anat Embryol 168:73–86
Goffinet AM (1984) Events governing organization of postmigratory neurons: studies on brain development in normal and reeler mice. Brain Res Rev 7:261–296
Goffinet AM, So K-F, Yamamoto M, Edwards M, Caviness VS Jr (1984) Architectonic and hodological organization of the cerebellum in reeler mutant mice. Dev Brain Res 16:263–76
Gravel C, Hawkes R (1990) Parasagittal organization of the rat cerebellar cortex: direct comparison of Purkinje cell compartments and the organization of the spinocerebellar projection. J Comp Neurol 291:79–102
Gravel C, Eisenman LM, Sasseville R, Hawkes R (1987) Parasagittal organization of the rat cerebellar cortex: direct correlation between antigenic Purkinje cell bands revealed by mabQ113 and the organization of the olivocerebellar projection. J Comp Neurol 265:294–310
Hancock MB (1986) Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat 175:343–352
Hawkes R (1992) Antigenic markers of cerebellar modules in the adult mouse. Biochem Soc Trans 20:391–395
Hawkes R, Leclerc N (1986) Immunocytochemical demonstration of topographic ordering of Purkinje cell axon terminals in the fastigial nuclei of the rat. J Comp Neurol 244:481–491
Hawkes R, Leclerc N (1987) Antigenic map of the rat cerebellar cortex: The distribution of parasagittal bands as revealed by monoclonal anti-Purkinje cell antibody mabQ113. J Comp Neurol 256:29–41
Hawkes R, Colonnier M, Leclerc N (1985) Monoclonal antibodies reveal sagittal banding in the rodent cerebellar cortex. Brain Res 333:359–365
Heckroth JA, Goldowitz D, Eisenman LM (1989) Purkinje cell reduction in the reeler mutant mouse: a quantitative immunohistochemical study. J Comp Neurol 279:546–555
Inoue Y, Maeda N, Kokubun T, Takayama C, Inoue K, Terashima T, Mikoshiba K (1990) Architecture of Purkinje cells of the reeler mutant mouse observed by immunohistochemistry for the inositol 1,4,5-trisphosphate receptor protein P400. Neurosci Res 8:189–201
Leclerc N, Gravel C, Hawkes R (1988) Development of parasagittal zonation in the rat cerebellar cortex: MabQ113 antigenic bands are created postnatally by the suppression of antigen expression in a subset of Purkinje cells. J Comp Neurol 273:399–420
Leclerc N, Dore L, Parent A, Hawkes R (1990) The compartmentation of the monkey and rat cerebellar cortex: Zebrin I and cytochrome oxidase. Brain Res 506:70–78
LeClerc N, Schwarting G, Herrup K, Hawkes RB, Yamamoto M (1992) Compartmentation in mammalian cerebellum: Zebrin II and P-path antibodies define three classes of sagittally organized bands of Purkinje cells. Proc Nat Acad Sci USA 89:5006–5010
Mariani J, Crepel F, Mikoshiba K, Changeux JP, Sotelo C (1977) Anatomical, physiological and biochemical studies of the cerebellum from reeler mutant mouse. Philos Trans R Soc Lond Biol 281:1–28
Matsushita M, Ragnarson B, Grant G (1991) Topographic relationship between sagittal Purkinje cell bands revealed by a monoclonal antibody to Zebrin I and spinocerebellar projections arising from the central cervical nucleus in the rat. Exp Brain Res 84:133–141
Meier H, Hoag WG (1962) The neuropathology of “reeler”, a neuromuscular mutation in mice. J Neuropath Exp Neurol 21:649–654
Mullen RJ (1977) Genetic dissection of the CNS with mutant-normal mouse and rat chimeras. Soc Neurosci Symp 2:47–65
Oberdick J, Shilling K, Smeyne RJ, Corbin JG, Bocchiaro C, Morgan JI (1993) Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron 10:1007–1018
Pinto-Lord MC, Evrard P, Caviness VS Jr (1982) Obstructed neuronal migration along radial glial fibers in the neocortex of the reeler mouse: a Golgi-EM analysis. Dev Brain Res 4:379–393
Rouse RV, Sotelo C (1990) Grafts of dissociated cerebellar cells containing Purkinje cell precursors organize into Zebrin I defined compartments. Exp Brain Res 82:401–407
Scott TG (1964) A unique pattern of localization within the cerebellum of the mouse. J Comp Neurol 122:1–8
Smeyne RJ, Oberdick J, Schilling K, Berrebi AS, Mugnaini E, Morgan JI (1991) Dynamic organization of developing Purkinje cells revealed by transgene expression. Science 254:719–721
Sotelo C, Wassef M (1991) Purkinje cell heterogeneity in four cerebellar mutations revealed by Zebrin I expression. Neurosci Abst 17:918
Sotelo C, Bourrat F, Triller A (1984) Postnatal development of the inferior olivary complex in the rat. II. Topographic organization of the immature olivocerebellar projection. J Comp Neurol 222:177–199
Tano D, Napieralski JA, Eisenman LM, Messer A, Plummer J, Hawkes R (1992) Novel developmental boundary in the cerebellum revealed by Zebrin expression in the Lurcher (Lc/+) mutant mouse. J Comp Neurol 323:128–136
Terashima T, Onoue K, Inoue Y, Yokoyama M, Mikoshiba K (1986) Observations on the cerebellum of normal-reeler mutant mouse chimera. J Comp Neurol 252:264–278
Underwood EE (1970) Quantitative stereology. Addison-Wesley Press, Reading, Pa
Voogd J (1969) The importance of fiber connections in the comparative anatomy of the mammalian cerebellum. In: Llinas R (ed) Neurobiology of cerebellar evolution and development. AMA, Chicago, pp 493–541
Voogd J, Gerrits NM, Marani E (1985) Cerebellum. In: The rat nervous system, vol 2. Academic Press, Sydney, pp 251–291
Wassef M, Sotelo C (1984) Asynchrony in the expression of guanosine 3′∶5′-phosphate-dependent protein kinase by clusters of Purkinje cells during the perinatal development of rat cerebellum. Neuroscience 13:1217–1241
Wassef M, Zanetta JP, Brehier A, Sotelo C (1985) Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol 111:129–137
Wassef M, Sotelo C, Cholley B, Brehier A, Thomasset M (1987) Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev Biol 124:379–389
Wassef M, Sotelo C, Thomasset M, Granholm A-C, Leclerc N, Rafrafi J, Hawkes R (1990) Expression of compartmentation antigen Zebrin I in cerebellar transplants. J Comp Neurol 294:223–234
Wassef M, Cholley B, Heizmann CW, Sotelo C (1992) Development of the olivocerebellar projection in the rat. II. Matching of the developmental compartmentations of the cerebellum and inferior olive through the projection map. J Comp Neurol 323:537–550
Wilson L, Sotelo C, Caviness VS Jr (1981) Heterologous synapses upon Purkinje cells in the cerebellum of the reeler mutant mouse: an experimental light and electron microscopic study. Brain Res 213:63–82
Yuasa S, Kitoh J, Oda S, Kawamura K (1993) Obstructed migration of Purkinje cells in the developing cerebellum of the reeler mutant mouse. Anat Embryol 188:317–329
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edwards, M.A., Leclerc, N., Crandall, J.E. et al. Purkinje cell compartments in the reeler mutant mouse as revealed by Zebrin II and 90-acetylated glycolipid antigen expression. Anat Embryol 190, 417–428 (1994). https://doi.org/10.1007/BF00235488
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235488