Summary
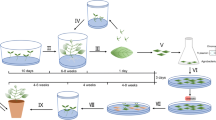
Twelve cultivars of Brassica juncea grown in different agroclimatic regions of the world were tested for their ability to regenerate in vitro from hypocotyl explants and, accordingly, were divided into three groups. One group of cultivars regenerated on MS medium supplemented with 2,4-D, BAP and with NAA, BAP combinations; another group regenerated only on MS with 2,4-D, BAP; and the third group showed very low regeneration on both of these combinations. Inclusion of silver nitrate in the medium was essential for high frequency of regeneration. In general, Indian cultivars were more responsive than the cultivars of CIS and Australian origin. Using the media optimal for regeneration and an Agrobacterium-based binary vector carrying hpt and gus-intron genes, conditions for genetic transformation of B. juncea hypocotyl explants were optimized. Transformation frequencies, identified by GUS staining at the initial stages of growth, were lower on MS medium with 2,4-D, BAP than on MS with NAA, BAP. Plants resistant to 20 μg/ml hygromycin were regenerated at a frequency of 11–36% from hypocotyl explants and were shown to be transformed by Southern blotting, GUS staining and progeny analysis.
Similar content being viewed by others
References
Barfield DG and Pua EC (1991) Gene transfer in Brassica juncea using Agrobacterium tumefaciens-mediated transformation. Plant Cell Rep. 10:308–314
Bonfils AE, Sproule A, Webb JA and Keller WA (1992) Plant regeneration from stem cortex explant and protoplast cultures of Brassica juncea (mustard). Plant Cell Rep. 11:614–617
Chi GL, Barfield DG, Sim GE and Pua EC (1990) Effect of AgNO3 and aminoethyoxyvinylglycine on in vitro shoot and root organogenesis from seedling explants of recalcitrant Brassica genotypes. Plant Cell Rep. 9:195–198
Deblaere R, Bytebier B, De Greve He, Debocck F, Schell J, Van Montagu M and Leemans J (1985) Efficient octapine Ti plasmid-derived vectors for Agrobacterium mediated gene transfers. Nucl. Acids Res. 13:4777–4788
De Block M, De Brouwer D and Tenning P (1989) Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 91:694–701
Dellaporta SL, Wood J and Hicks JB (1983) A plant molecular DNA minipreparation version: II Plant Mol. Biol. Reporter 1:19–21
Fazekas GA, Sedmach PA and Palmer MV (1986) Genetic and environmental effects on in vitro shoot regeneration from cotyledon explants of Brassica juncea. Plant Cell Tiss. Org. Cult. 6:183–187
George L and Rao PS (1980) In vitro regeneration of mustard plants (Brassica juncea var. Rai-5) from cotyledon explants from nonirradiated, irradiated and mutagen treated seed. Ann. Bot. 46:107–112
Jain RK, Chowdhary JB, Sharma DR and Friedt W (1988) Genotypic and media effects on plant regeneration from cotyledon explant cultures of some Brassica species. Plant Cell Tiss. Org. Cult. 14:197–206
Jefferson RA (1987) Assaying chimeric genes in plant: the GUS gene fusion system. Plant Mol. Biol. Reporter 5:387–405
Kirti PB and Chopra VL (1989) A simple method of inducing somatic embryogenesis in Brassica juncea (L.) Czern & Coss. Plant Breed. 102:73–78
Mariani C, De Beuckeleer M, Truettner J, Leemans J and Goldberg R (1990). Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 347:737–741.
Mariani C, Gossele V, De Beuckeleer M, De Block M, Goldberg R, De Greef W and Leemans J (1992). A chimeric ribonuclease inhibitor gene restores fertility to male sterility plants. Nature 357:384–387.
Mathews H, Bharathan N, Litz RE, Narayanan KR, Rao PS and Bhatia CR (1990) Transgenic plants of mustard Brassica juncea (L.) Czern and Coss. Plant Sci. 72:245–252
Mattanovich D, Ruker F, Machado AC, Laimer M, Regner F, Steinkellner H, Himmler G and Katinger H (1989) Efficient transformation of Agrobacterium spp. by electroporation. Nucl. Acids Res. 17:6747
Moloney MM, Walker JM and Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep. 8:238–242
Mukhopadhyay A, Topfer R, Pradhan AK, Sodhi YS, Steinbiss HH, Schell J and Pental D (1991) Efficient regeneration of Brassica oleracea hypocotyl protoplasts and high frequency genetic transformation by direct DNA uptake. Plant Cell Rep. 10:375–379
Mukhopadhyay A, Arumugum N, Nanda Kumar PBA, Pradhan AK, Gupta V and Pental D (1992) Agrobacteriumm mediated genetic transformation of oilseed Brassica campestris: Transformationfrequency is strongly influenced by the mode of shoot regeneration. Plant Cell Rep. 11:506–513
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 15:473–493
Radke SE, Turner JC, Facciotti D (1992) Transformation and regeneration of Brassica rapa using Agrobacterium tumefaciens. Plant Cell Rep 11:499–505
Sharma KK, Bhojwani SS and Thorpe TA (1990) Factors affecting high frequency differentiation of shoots and roots from cotyledon explants of Brassica juncea (L.) Czern. Plant Sci. 66:247–253
Topfer R, Matzeit V, Gronenborn B, Schell J and Steinbiss HH (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucl. Acids Res. 14:5890
Vancanneyt G, Schmidt R, O'connor-Sanchez A, Willmitzer L and Rocha-Sosa M. (1990) Construction of an intron-containing marker gene: Splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium mediated plant transformation. Mol. Gen. Genet. 220:245–250
Vaughan JG, Hemingway JS, Schofield HL (1963) Contributions of a study on variation in Brassica juncea Coss and Czern. J. Linn. Soc. (Bot), 58:435–447
Author information
Authors and Affiliations
Additional information
Communicated by C. Quiros
Rights and permissions
About this article
Cite this article
Pental, D., Pradhan, A.K., Sodhi, Y.S. et al. Variation amongst Brassica juncea cultivars for regeneration from hypocotyl explants and optimization of conditions for Agrobacterium-mediated genetic transformation. Plant Cell Reports 12, 462–467 (1993). https://doi.org/10.1007/BF00234713
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00234713