Abstract
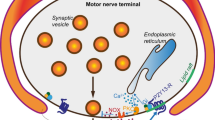
Sarcoplasmic reticulum (SR) vesicles were prepared from either canine or sheep heart and fused into lipid bilayers to study their ionic channels. A 92±5 pS anion-selective channel was recorded in asymmetric 50 mm trans/250 mm cis CsCl buffer system. Reversal potentials and theoretical equilibrium potentials for Cl− ions obtained under various experimental conditions allowed us to confirm the Cl− selectivity of this SR channel. The majority (69%) of channel recordings (n = 45) displayed steady-state kinetics and a slight voltage dependency of the open probability. However, 31% of the channels inactivated after their incorporation. We now report that the channel might be reactivated by depolarizing voltage steps. Furthermore, the use of either PKA or PKG in association with adequate phosphorylating buffers lengthens the deactivation process at the end of the voltage pulses, but does not prevent the inactivation. It was assumed that the change in gating mode was due to a voltage-sensitive association/dissociation mechanism with a phosphorylated protein of the SR membrane such as phospholamban (PL). We demonstrated that a specific monoclonal antibody raised against canine PL inhibited the activity of the channel and prevented its reactivation by depolarizing steps. 400 to 800 ng/ml of Anti-PL Ab consistently and sequentially turned off the channel activities. In contrast, heat inactivated Anti-PL Ab had no effect. We propose that phospholamban may be a primer of the SR Cl− channel whereby Cl− anions would play the role of counter-charge carrier during rapid Ca2+ release and Ca2+ uptake by the SR.
Similar content being viewed by others
References
Caillé, J.P. 1986. Intracellular chloride activity in rabbit papillary muscle: effect of serum. Can. J. Physiol. Pharmacol. 64:1381–1384
Coulombe, A., Coraboeuf, E. 1992. Large-conductance chloride channels of new-born rat cardiac myocytes are activated by hypotonic media. Pfluegers Arch. 422:143–150
Davis, B.A., Edes, I., Gupta, R.C., Young, E.F., Kim, H.W., Steenaart, N.A., Szymanska, G., Kranias, E.G. 1990. The role of phospholamban in the regulation of calcium transport by cardiac sarcoplasmic reticulum. Mol. and Cell Biochem. 99(2):83–88
Decrouy, A., Juteau, M., Rousseau, E. 1994. A phosphorylated membrane component modulates the voltage dependent kinetics of the SR Cl− channel. Biophys. J. 66(2):W-Pos369
Doroshenko, P., Penner, R., Neher, E. 1991. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTPγS. S. J. Physiol. 436:711–724
Edes, I., Kranias, E.G. 1990. Phospholamban and troponin I are substrates for protein kinase C in vitro but not in intact beating guinea pig hearts. Circ. Res. 67:394–400
Fabiato, A. 1988. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solution containing multiple metals and ligands. In: Methods in Enzymology, Membrane. S. Fleischer and B. Fleischer editors, pp. 378–417. Academic Press, Orlando, FL
Fisher, D.J., Tate, C.A., Philips, S. 1992. The role of dicarboxylic anion transport in the slower Ca2+ uptake in fetal cardiac sarcoplasmic reticulum. Pediatr. Res. 32:664–668
Hals, G.D., Palade, P.T. 1990. Different sites control voltage dependence and conductance of sarcoball anion channel. Biophys J. 57(5):1037–1047
Hill, J.A., Coronado, R. Jr., Strauss, H.C. 1990. Open-channel subconductance state of K+ channel from cardiac sarcoplasmic reticulum. Am J Physiol 258:H159–164
Kawano, S., Nakamura, F., Tanaka, T., Hiroaka, M. 1992 Cardiac sarcoplasmic reticulum chloride channels regulated by protein Kinase A. Circ. Res. 71:585–589
Kawano, S., Hiraoka, M. 1993. Protein Kinase A-activated chloride channel is inhibited by the Ca2+-calmodulin complex in cardiac sarcoplasmic reticulum. Circ. Res. 73:751–757
Kotera, T., Brown, P.D. 1994. Cl− current activation in choroid plexus epithelial cells involves a G protein and protein kinase A. Am. J. Physiol. 266:C536-C540
Lowry, O.H., Rosebrough, N.J., Fan, A.L., Rendall, R.J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275
Lugnier, C., Muller, B., LeBec, A., Beaudry, C., Rousseau, E. 1993. Characterization of indolidanand rolipram-sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J. Pharmacol. and Exper. Ther. 265(3):1142–1151
McKinley, D., Meissner, G. 1978. Evidence for a K+, Na+ permeable channel in sarcoplasmic reticulum. J. Membrane Biol. 44:159–186
Meissner, G., Henderson, J. 1987. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide and calmodulin. J. Biol. Chem. 262: 3065–3073
Miller, C., Raker, E. 1976. Ca2+ induced fusion of fragmented SR with artificial bilayer. J. Membrane Biol. 30:283–300
Morris, G.L., Cheng, H.-C., Colyer, J., Wang, J.H. 1991. Phospholamban regulation of cardiac sarcoplasmic reticulum (Ca2+Mg2+)ATPase. J. Biol. Chem. 266:11270–11275
Movsesian, M.A., Palmer, E.O., Palmer, B.L., Cheung, J.Y., Moore, R.L. 1994. Effects of isoproterenol on intracellular free [Ca2+] during relaxation in rat cardiac myocytes. Biophys. J. 66(2):M-Pos383
Nagel, G., Hwang, T.-C., Nastiuk, K.L., Nairn, A.C., Gadsby, D.C. 1992. The protein Kinase A-regulated cardiac Cl− channel resembles the cystic fibrosis transmembrane conductance regulator. Nature 360:81–84
Oetliker, H. 1982. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J. Muscle Res. Cell. Motil. 3:247–272
Raeymaekers, L., Hofmann, F., Casteels, R. 1988. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in iso-lated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem. J. 252:269–273
Rousseau, E., Smith, J.S., Henderson, J.S., Meissner, G. 1986. Single channel and 45Ca2+ flux measurements of the cardiac sarcoplasmic reticulum calcium channel. Biophys. J. 50:1009–1014
Rousseau, E., Roberson, M., Meissner, G. 1988. Properties of single chloride selective channel from sarcoplasmic reticulum. Eur. Biophys. J. 16:143–151
Rousseau, E. 1989. Single chloride-selective channel from cardiac sarcoplasmic reticulum studied in planar lipid bilayers. J. Membrane Biol. 110:39–47
Rousseau, E., Chabot, H., Beaudry, C., Muller, B. 1992. Reconstitution and regulation of cation-selective channels from cardiac sarcoplasmic reticulum. Mol. and Cell Biochem. 114:109–117
Szymanska, G., Kim, H.W., Cuppoletti, J., Kranias, E.G. 1992. Regulation of the skeletal sarcoplasmic reticulum Ca2+-ATPase by phospholamban and negatively charged phospholipids in reconstituted phospholipid vesicles. Mol. Cell Biochem. 114:65–71
Tang, J.M., Wang, J., Eisenberg, R.S. 1989. K+-selective channel from sarcoplasmic reticulum of split lobster muscle fibers. J. Gen. Physiol 94:261–278
Tanifuji, M., Sokabe, M., Kasai, M. 1987. An anion channel of sarcoplasmic reticulum incorporated into planar lipid bilayers: single-channel behavior and conductance properties. J. Membrane Biol. 99:103–111
Tinker, A., Lindsay, A.R.G., Williams, A.J. 1993. Cation conduction in the cardiac sarcoplasmic reticulum calcium-release channel under physiological and pathophysiological conditions. Cardiovasc. Res. 27:1820–1825
Author information
Authors and Affiliations
Additional information
This study was supported by grants from HSFC and CRM of Canada. A. Decrouy is a recipient of an institutional postdoctoral fellowship and E. Rousseau is a FRSQ Scholar. The author would like to thank Dr. E. Kranias for her comments and relevant suggestions for the use of the monoclonal anti-PL antibody as well as Mrs. M. Picher and Miss S. Proteau for thier skillful technical assistance.
Rights and permissions
About this article
Cite this article
Decrouy, A., Juteau, M. & Rousseau, E. Examination of the role of phosphorylation and phospholamban in the regulation of the cardiac sarcoplasmic reticulum Cl− channel. J. Membarin Biol. 146, 315–326 (1995). https://doi.org/10.1007/BF00233951
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00233951