Abstract
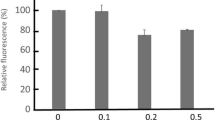
Harvesting MDCK cells with trypsin-EDTA reduces potassium currents (I K) to a mere 10%, presumably by hydrolysis of K+ channels, but replating at confluence restores them in 12–18 hr, through a process that requires transcription, translation and exocytic fusion of intracellular membrane vesicles to the plasma membrane (Ponce & Cereijido, 1991; Ponce et al., 1991a). In the present work we find that this restoration of I K also requires cell-cell contacts and the presence of 1.8 mm Ca2+. The role of extracellular Ca2+ may be substituted by 2.0 μm TRH, 10 nm PMA or 200 μg/ml DiC8, drags that stimulate the system of phospholipase C (PLC) and protein kinase C (PKC). Conversely, the recovery of I K triggered by Ca-dependent contacts can be blocked by 110 μm neomycin, 2.0 μm H7, and 250 nm staurosporine, inhibitors of PLC and PKC. These results suggest that the expression of new K+ channels depends on Ca2+-activated contacts with neighboring cells and that the information is conveyed through PLC and PKC, a process in keeping with changes in its enzymatic activity and cellular distribution of PKC. Plasma membrane is also reduced and restored upon harvesting and replating, and depends on Ca2+-activated contracts. However, the effects of the chemicals tested on I K differ from the ones they elicit on the recovery of plasma membrane, suggesting that cells can independently regulate their population of K+ channels and the surface of their membrane.
Similar content being viewed by others
References
Balda, M.S., González-Mariscal, L., Contreras, R.G., Cereijido, M. 1991. Assembly and sealing of tight junctions: Possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J. Membrane Biol. 122:193–202
Balda, M.S., González-Mariscal, L., Matter, K., Cereijido, M., Anderson, J.M. 1993. Assembly of the tight junction: the role of diacylglycerol. J. Cell Biol. 123:293–302
Bolívar, J.J., Cereijido, M. 1987. Voltage and Ca2+-activated K+ channel in cultured epithelial cells (MDCK). J. Membrane Biol. 97:43–51
Cereijido, M., Ehrenfeld, J., Meza, I., Martínez-Palomo, A. 1980. Structural and functional membrane polarity in cultured monolayers of epithelioid MDCK cells. J. Membrane Biol. 52:147–159
Cereijido, M., Robbins, E.S., Dolan, W.J., Rotunno, C.A., Sabatini, D.D. 1978. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 77:853–880
Contreras, R.G., Avila, G., Gutiérrez, C., Bolívar, J.J., Gonźalez-Mariscal, L., Darzon, A., Beaty, G., Rodríguez-Boulan, E., Cereijido, M. 1989. Repolarization of Na+-K+ pumps during establishment of epithelial monolayers. Am. J. Physiol. 257:C896-C905
Contreras, R.G., González-Mariscal, L., Balda, M.S., García-Villegas, M.R., Cereijido, M. 1992a. The role of calcium in the making of a transporting epithelium. NIPS 7:105–108
Contreras, R.G., Miller, J.H., Zamora, M., González-Mariscal, L., Cereijido, M. 1992b. Interaction of calcium with plasma membrane of epithelial (MDCK) cells during junction formation. Am. J. Physiol. 263:C313-C318
Friedrich, F., Paulmichl, M., Kolb, H.A., Lang, F. 1988. Inward rectifier K+ channels in renal epithelioid cells (MDCK) activated by serotonin. J. Membrane Biol. 106:149–155
González-Mariscal, L., Chávez de Ramírez, B., Cereijido, M. 1985. Tight junction formation in cultured epithelial cells (MDCK). J. Membrane Biol. 86:113–125
González-Mariscal, L., Contreras, R.G., Bolívar, J.J., Ponce, A., Chávez de Ramírez, B., Cereijido, M. 1990. Role of calcium in tight junction formation between epithelial cells. Am. J. Physiol. 259:C978-C986
Gumbiner, B., Simons, K. 1987. The role of uvomorulin in the formation of epithelial occluding junctions. In: Junctional Complexes of Epithelial Cells. pp. 169–186. Wiley, Chichester, UK
Knight, D.E., Sugden, D., Baker, P.F. 1988. Evidence implicating protein kinase C in exocytosis from electropermeabilized bovine chromaffin cells. J. Membrane Biol. 104:21–34
Kolb, H.A., Paulmichl, M., Lang, F. 1987. Epinephrine activates outward rectifying K+ channels in Madin-Darby canine kidney cells. Pfluegers Arch. 408:548–591
Kraft, A.S., Anderson, W.B. 1983. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature 301:621–623
Lang, F., Defregger, M., Paulmichl, M. 1986. Apparent chloride conductance of subconfluent Madin Darby canine kidney cells. Pfluegers Arch. 407:158–162
Martin, T.F.J., Lucas, D.O., Bajjaleih, S.M., Kowalchyk, J.A. 1986. Thyrotropin-releasing hormone activates a Ca++-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by cholera and pertussis toxin insensitive mechanism. J. Biol. Chem. 261:2918–2927
May, W.S., Lapetina, E.G., Cuatrecasas, P. 1986. Intracellular activation of protein kinase C and regulation of surface transferrin receptor by diacylglycerol is a spontaneous reversible process that is associated with rapid formation of phosphatidic acid. Proc. Natl. Acad. Sci. USA 83:1281–1284
Myers, C.L., Lazo, J.S., Pitt, B.R. 1989. Translocation of protein kinase C is associated with inhibition of 5-HT uptake by cultured endothelial cells. Am. J. Physiol. 257:L253-L258
Ponce, A., Bolívar, J.J., Vega, J., Cereijido, M. 1991a. Synthesis of plasma membrane and potassium channels in epithelial (MDCK) cells. Cell Physiol. Biochem. 1:195–204
Ponce, A., and Cereijido, M. 1991. Polarized distribution of cation channels in epithelial cells. Cell Physiol. Biochem. 1:13–23
Ponce, A., Contreras, R.G., Cereijido, M. 1991b. Polarized distribution of chloride channels in epithelial cells (MDCK). Cell Physiol. Biochem. 1:160–169
Stefani, E., Cereijido, M. 1983. Electrical properties of cultured epithelioid cells (MDCK). J. Membrane Biol. 73:177–184
Tamaoki, T., Nomoto, H., Takahashi, I., Kato, Y., Morimoto, M., Tomita, F. 1986. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem. Biophys. Res. Commun. 135:397–402
Vega-Salas, D.E., Salas, P.J., Rodríguez-Boulan, E. 1987. Modulation of the expression of an apical plasma membrane protein of Madin-Darby Canine Kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J. Cell. Biol. 104:1249–1259
Yasuda, I., Kishimoto, A., Tanaka, S., Tominaga, M., Sakurai, A., Nishizuka, Y. 1990. A synthetic peptide substrate for selective assay of protein kinase C. Biochem. Biophys. Res. Commun. 166:1220–1227
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Talavera, D., Ponce, A., Fiorentino, R. et al. Expression of potassium channels in epithelial cells depends on calcium-activated cell-cell contacts. J. Membarin Biol. 143, 219–226 (1995). https://doi.org/10.1007/BF00233450
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00233450