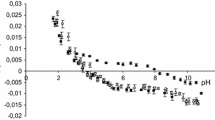
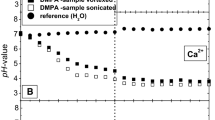
Abstract
The rate of change of internal pH and transmembrane potential has been monitored in liposomes following the external addition of various cation salts. Oleic acid increases the transmembrane movement of H+ following the imposition of a K+ gradient. An initial fast change in internal pH is seen followed by a slower rate of alkalinization. High concentrations of the fatty acid enhance the rate comparable to that seen in the presence of nigericin in contrast to the effect of FCCP (carbonyl cyanide p-(tri-fluoromethoxy)phenyl hydrazone) which saturates at an intermediate value. The ability of nonesterified fatty acids to catalyze the movement of cations across the liposome membrane increases with the degree of unsaturation and decreases with increasing chain length. Li and Na salts cause a similar initial fast pH change but have less effect on the subsequent slower rate. Similarly, the main effect of divalent cation salts is on the initial fast change. The membrane potential can enhance or inhibit cation transport depending on its polarity with respect to the cation gradient. It is concluded that nonesterified fatty acids have the capability to complex with, and transport, a variety of cations across phospholipid bilayers. However, they do not act simply as proton/cation exchangers analogous to nigericin nor as protonophores analogous to FCCP. The full cycle of ionophoric action involves a combination of both functions.
Similar content being viewed by others
References
Bashford, C.L., Chance, B., Prince, R.C. 1979. Oxonol dyes as monitors of membrane potential: Their behaviour in photosynthetic bacteria. Biochim. Biophys. Acta 545:46–57
Borst, P., Loos, J.A., Christ, E.J., Slater, E.C. 1962. Uncoupling activity of long-chain fatty acids. Biochim. Biophys. Acta 62:509–518
Brown, G.C., Brand, M.D. 1991. On the nature of the mitochondrial proton leak. Biochim. Biophys. Acta 1059:55–62
Burt, J.M., Massey, K.D., Minnich, B.N. 1991. Uncoupling of cardiac cells by fatty acids: structure-activity relationships. Am. J. Physiol. 260:C439–448
Casey, R.P. 1984. Membrane reconstitution of the energy-conserving enzymes of oxidative phosphorylation. Biochim. Biophys. Acta 768:319–347
Cooper, C.E., Bruce, D., Nicholls, P. 1990. Use of oxonol V as a probe of membrane potential in proteoliposomes containing cytochrome oxidase in the submitochondrial orientation. Biochemistry 29: 3859–3865
Cooper, C.E., Wrigglesworth, J.M., Nicholls, P. 1990. The mechanism of potassium movement across the liposomal membrane. Biochem. Biophys. Res. Commun. 173:1008–1012
Cowan, J.C., Vaughan-Williams, R.N. 1977. The effect of palmitate on intracellular potentials recorded from Langendorff-perfused guinea-pig hearts in normoxia and hypoxia and during perfusion at reduced rate of flow. J. Mol. Cel. C. 9:327–342
Deamer, D.W., Bramhall, J. 1986. Permeability of lipid bilayers to water and ionic solutes. Chem. Phys. Lipids 40:167–188
Deamer, D.W., Nichols, J.W. 1983. Proton-hydroxide permeability of liposomes. Proc. Natl. Acad. Sci. USA 80:165–168
Deamer, D.W., Nichols, J.W. 1989. Proton flux mechanisms in model and biological membranes. J. Membrane Biol. 107:91–103
Eisenberg, M., Gresalfi, T., Riccio, T.M., McLaughlin, S. 1979. Adsorption of monovalent cations to membranes containing negative phospholipids. Biochemistry 18:5213–5223
Ermakov, Y.A. 1990. The determination of binding site density and association constants for monovalent cation adsorption onto liposomes made from mixtures of zwitterionic and charged lipids. Biochim. Biophys. Acta 1023:91–97
Gutknecht, J. 1988. Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J. Membrane Biol. 106:83–93
Jandacek, R.J., Broering, W.B. 1989. X-ray diffraction study of sodium soaps of monounsaturated and polyunsaturated fatty acids. Lipids 24:1008–1013
Kamp, F., Hamilton, J.A. 1992. pH gradients across phospholipid membranes caused by fast flip-flop on unionized fatty acids. Proc. Natl. Acad. Sci. USA 89:11367–11370
Kidooka, M., Matsuda, M., Handa, J. 1987. Ca2+ antagonist and protection of the brain against ischemia. Effects of nicardipine on free fatty acid liberation in the ischemic brain in rats. Surg. Neurol. 28:437–444
Klug, G.A., Krause, J., Ostlund, A.-K., Knoll, G., Brdiczka, D. 1984. Alterations in liver mitochondrial function as a result of fasting and exhausive exercise. Biochim. Biophys. Acta 764:272–282
Krishnamoorthy, G., Hinkle, P.C. 1984. Non-ohmic proton conductance of mitochondria and liposomes. Biochemistry 23:1640–1645
Labonia, N., Muller, M., Azzi, A. 1988. The effect of non-esterified fatty acids on the proton-pumping cytochrome c oxidase reconstituted into liposomes. Biochem. J. 254:139–145
Macri, F., Vianello, A., Braidot, E., Zancani, M. 1991. Free fatty acids dissipate proton electrochemical gradients in pea stem microsomes and submitochondrial particles. Biochim. Biophys. Acta 1050:249–255
McLaughlin, A.C. 1982. Phosphorus-31 and carbon-13 nuclear magnetic resonance studies of divalent cation binding to phosphatidylserine membranes: Use of cobalt as a paramagnetic probe. Biochemistry 21:4879–4885
McLaughlin, S.G.A., Dilger, J.P. 1980. Transport of protons across membranes by weak acids. Physiol. Rev. 60:825–863
Newsholme, E.A., Leech, A.R. 1983. Biochemistry for the Medical Sciences. John Wiley & Sons, Chichester
Nichols J.W., Hill, M.W., Bangham, A.D., Deamer, D.W. 1980. Measurement of net proton-hydroxyl permeability of large unilamellar liposomes with the fluorescent pH probe, 9-aminoascridine. Biochim. Biophys. Acta 596:393–403
Nobes, C.D., Hay, W.W., Brand, M.D. 1990. The mechanism of stimulation of respiration by fatty acids in isolated hepatocytes. J. Biol. Chem. 265:12910–12915
Rossignol, M., Thomas, P., Grignon, C. 1982. Proton permeability of liposomes from natural phospholipid mixtures. Biochim. Biophys. Acta 684:195–199
Rottenberg, H., Hashimoto, K. 1986. Fatty acid uncoupling of oxidative phosphorylation in rat liver mitochondria. Biochemistry 25:1747–1755
Serhan, C., Andersop, P., Goodman, E., Dunham, P., Weissmann, G. 1981. Phosphatidate and oxidized fatty acids are calcium ionophores. J. Biol. Chem. 256:2736–2741
Simpson, R.J., Peters, T.J. 1987. Transport of Fe+ across lipid bilayers: Possible role of free fatty acids. Biochim. Biophys. Acta 898:187–195
Simpson, R.J., Moore, R., Peters, T.J. 1988. Significance of non-esterified fatty acids in iron uptake by intestinal brush-border membrane vesicles. Biochim. Biophys. Acta 941:39–47
Spooner, P.J.R., Gantz, D.L., Hamilton, J.A., Small, D.M. 1990. The distribution of oleic acid between chylomicron-like emulsions, phospholipid bilayers and serum albumin. J. Biol. Chem. 265: 12650–12655
Thiel, C., Kadenbach, B. 1989. Influence of non-esterified fatty acids on respiratory control of reconstituted cytochrome-c oxidase. FEBS Lett. 251:270–274
Wilschut, J., Scholma, J., Eastman, S.J., Hope, M.J., Cullis, P.R. 1992. Ca2+-induced fusion of phospholipid vesicles containing free fatty acids: Modulation by transmembrane pH gradients. Biochemistry 31:2629–2636
Wojtczak, L. 1976. Effect of long-chain fatty acids and acyl-CoA on mitochondrial permeability, transport and energy coupling processes. J. Bioenerg. Biomembr. 8:293–311
Wrigglesworth, J.M., Cooper, C.E., Sharpe, M.A., Nicholls, P. 1990. The proteoliposomal steady state: Effect of size, capacitance and membrane permeability on cytochrome-oxidase induced ion gradients. Biochem. J. 270:109–118
Wrigglesworth, J.M., Wooster, M.S., Elsden, J., Danneel, H.-J. 1987. Dynamics of proteoliposome formation: Intermediate states during detergent dialysis. Biochem. J. 246:737–744
Author information
Authors and Affiliations
Additional information
The authors would like to thank P. Nicholts (Brock University, Canada) for helpful discussions. M.A.S. received a Science and Engineering Research Council studentship and C.E.C. acknowledges the award of a King's College London fellowship followed by a Medical Research Council Training Fellowship.
Rights and permissions
About this article
Cite this article
Sharpe, M.A., Cooper, C.E. & Wrigglesworth, J.M. Transport of K+ and other cations across phospholipid membranes by nonesterified fatty acids. J. Membarin Biol. 141, 21–28 (1994). https://doi.org/10.1007/BF00232870
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00232870