Summary
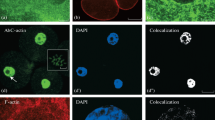
Fully polymerization-competent fluorescein-labeled actin from skeletal muscle was microinjected into both normal moving and experimentally treated Amoeba proteus. Its intracellular distribution was followed by integral image intensification of the fluorescence on a television screen and compared with controls injected with rhodamine-labeled serum albumin. The labeled actin was incorporated into the endogenous actin pool and exhibited a characteristic redistribution depending on the cellular morphology. Increased amounts of labeled actin could be detected within a thin layer separating the hyalo- and granuloplasm or running immediately beneath the plasma membrane when hyaloplasmic regions were absent. The topography of the fluorescent layer demonstrated in living cells is in agreement with the cortical microfilament layer described ultrastructurally recently in corresponding cells. The combined results emphasize the important role of the cortical filament layer in both morphogenetic processes (e.g., hyalo-granuloplasm separation or changes in cell shape) and motive force generation for cytoplasmic streaming and amoeboid movement.
Similar content being viewed by others
References
Allen RD (1961) A new theory of ameboid movement and protoplasmic streaming. Exp Cell Res (Suppl) 8:17–31
Allen RD, Allen NSt (1978) Cytoplasmic streaming in amoeboid movement. Ann Rev Biophys Bioeng 7:469–495
Comly LT (1973) Microfilaments in Chaos carolinensis: membrane association, distribution and heavy meromyosin binding in the glycerinated cell. J Cell Biol 58:230–237
Condeelis JS, Taylor DL, Moore PL, Allen RD (1976) The mechanochemical basis of amoeboid movement. II. Cytoplasmic filament stability at low divalent cation concentrations. Exp Cell Res 101:134–142
Goldacre RJ (1956) The regulation of movement and polar organization in amoeba by intracellular feedback. Proc 1st Intern Congr Cybernetics:715–725
Goldacre RJ, Lorch IJ (1950) Folding and unfolding of protein molecules in relation to cytoplasmic streaming, amoeboid movement and osmotic work. Nature 166:487–499
Goldman R, Pollard T, Rosenbaum J (1976) Cell Motility. Cold Spring Harbor Laboratory, Vol 1–3
Grebecka L (1978a) Frontal cap formation and origin of monotactic forms of Amoeba proteus under culture conditions. Acta Protozool 17:191–202
Grebecka L (1978b) Micrurgical experiments on the frontal cap of monotactic forms of Amoeba proteus. Acta Protozool 17:203–212
Grebecka L, Hrebenda B (1978) Dynamics of the cortical layer in moving Amoeba proteus. Acta Protozool 18:143–144
Grebecki A (1978) Organization of motory functions in amoebae and in slime moulds plasmodia. Acta Protozool 18:43–47
Haberey M (1973) Räumliche Anordnung von Plasmafilamenten bei Thecamoeba spaeronucleolus. Cytobiologie 8:61–75
Haberey M, Stockem W (1971) Amoeba proteus: Morphologie, Zucht und Verhalten. Mikrokosmos 60:33–42
Hauser M (1978) Demonstration of membrane-associated and oriented microfilaments in Amoeba proteus by means of a Schiff base/glutaraldehyde fixative. Cytobiologie 18:95–106
Hrebenda B, Grebecka L (1978) Ultrastructure of the frontal cap of monotactic forms of Amoeba proteus. Cytobiologie 17:62–72
Jahn TL, Bovee EC (1969) Protoplasmic movements within cells. Physiological Reviews 49:793–861
Komnick H, Stockem W, Wohlfarth-Bottermann KE (1973) Cell motility: Mechanisms in protoplasmic streaming and amoeboid movement. Int Rev Cytol 34:169–249
Korn ED, Wright PL (1973) Macromolecular composition of an amoeba plasma membrane. J Biol Chem 248:439–447
Korohoda W, Stockem W (1975a) On the nature of hyaline zones in the cytoplasm of Amoeba proteus. Microsc Acta 77:129–141
Kreis ThE, Winterhalter KH, Birchmeier W (1979) In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci USA 76:3814–3818
Mast SO (1926) Structure, movement, locomotion and stimulation in Amoeba. J Morphol 41:347–425
Pollard TD, Korn ED (1973) Electron microscopic identification of actin associated with isolated amoeba plasma membranes. J Biol Chem 248:448–450
Rinaldi R A, Opas M, Hrebenda B (1975) Contractility of glycerinated Amoeba proteus and Chaos chaos. J Protozool 22:286–292
Stockem W (1978) Cell surface morphology and activity in Amoeba proteus and Physarum polycephalum. Acta Protozool 18:33–42
Stockem W, Gawlitta W, Weber K, Wehland J (in preparation) Structure and function of the cortical filament layer in Amoeba proteus
Stockem W, Weber K, Wehland J (1978) The influence of microinjected phalloidin on locomotion, protoplasmic streaming and cytoplasmic organization in Amoeba proteus and Physarum polycephalum. Cytobiologie 18:114–131
Taylor DL (1977) The contractile basis of amoeboid movement. IV. The visco-elasticity and contractility of amoeba cytoplasm in vivo. Exp Cell Res 105:413–426
Taylor DL, Condeelis JS (1979) Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol 56:57–144
Taylor DL, Wang YL (1978) Molecular cytochemistry: Incorporation of fluorescently labeled actin into living cells. Proc Natl Acad Sci USA 75:857–861
Taylor DL, Moore PL, Condeelis JS, Allen RD (1976a) The mechanochemical basis of amoeboid movement. I. Ionic requirements for maintaining visco-elasticity and contractility of amoeba cytoplasm. Exp Cell Res 101:127–133
Taylor DL, Rhodes JA, Hammond SA (1976b) The contractile basis of amoeboid movement. II. Filament formation in motile extracts and plasmalemma-ectoplasm ghosts. J Cell Biol 70:123–143
Taylor DL, Condeelis JS, Moore PL, Allen RD (1973) The contractile basis of amoeboid movement. I. The chemical control of motility in isolated cytoplasm. J Cell Biol 59:378–394
Wang YL, Taylor DL (1979) Distribution of fluorescently labeled actin in living sea urchin eggs during early development. J Cell Biol 81:672–679
Wehland J, Weber K (in press) Distribution of fluorescently labeled actin and tropomyosin after microinjection in living tissue culture cells as observed with TV-image intensification. Exp Cell Res
Wehland J, Osborn M, Weber K (1977) Phalloidin induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth. Proc Natl Acad Sci USA 74:5613–5617
Wehland J, Stockem W, Weber K (1978) Cytoplasmic streaming in Amoeba proteus is inhibited by the actin specific drug phalloidin. Exp Cell Res 115:451–454
Wehland J, Weber K, Gawlitta W, Stockem W (1979) Effects of the actin-binding protein DNAase I on cytoplasmic streaming and ultrastructure of Amoeba proteus. Cell Tissue Res 199:353–372
Wieland T (1977) Modification of actins by phallotoxins. Naturwissenschaften 64:303–309
Author information
Authors and Affiliations
Additional information
W. Gawlitta was supported by a fellowship (No. 21619) from the Konrad-Adenauer-Stiftung
The authors wish to thank Dr. Mary Osborn for reading the manuscript
Rights and permissions
About this article
Cite this article
Gawlitta, W., Stockem, W., Wehland, J. et al. Organization and spatial arrangement of fluorescein-labeled native actin microinjected into normal locomoting and experimentally influenced Amoeba proteus . Cell Tissue Res. 206, 181–191 (1980). https://doi.org/10.1007/BF00232762
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00232762