Summary
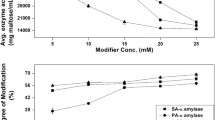
Modification of liquefying α-amylase by diethylpyrocarbonate or its photo-oxidation in the presence of rose bengal caused rapid loss of enzyme activity. The photo-oxidation followed pseudo-first-order kinetics giving maximal value at pH 8.0. The photo-oxidized enzyme showed a characteristic increase in absorbance at 250 nm which was directly proportional to the extent of inactivation. Diethylpyrocarbonate at low concentration at pH 6.0 and 30 ° C completely inactivated a-amylase. Inactivation followed pseudo-first-order kinetics. The reaction order with respect to inactivation by diethylpyrocarbonate was one, thus indicating modification of a single histidine per mole of the enzyme. Diethylpyrocarbonate-modified enzyme showed increased absorbance at 240 nm which was reversed completely upon treatment with NH2OH at 30 °C for 16 hr. Calculating the histidine residues being modified from the increase in absorbance at 240 nm showed that three residues were ethoxyformylated on treatment with diethylpyrocarbonate, of which only one was found at the active site. Substrate and competitive inhibitor protects the enzyme against both, photo-oxidation, and modification by diethylpyrocarbonate, confirming that histidine plays an essential role at the α-amylase active site.
Similar content being viewed by others
References
Robyt J, French D: Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys 100:451–467, 1963.
Thoma JA, Brothers C, Spradlin J: Subsite mapping of enzymes. Studies on Bacillus sublilis amylase. Biochemistry 4:1768–1775, 1970.
Iwasa S, Aosmisa H, Hiromi K, Hatano H: Subsite affinities of bacterial liquefying α-amylase evaluated from the rate parameters of linear substrates. J Biochem 75:969–978, 1974.
Ono S, Hiromi K, Yoshikawa Y: Kinetics of hydrolytic reaction catalyzed by crystalline bacterial α-amylase. Bull Chem Soc Japan 31:957–962, 1958.
Aosluma A, Manabe T, Hiromi K, Hakano H: Effect of photo-oxidation on bacterial liquefying α-amylase dependent on the degree of polymerization of linear substrate. Biochim Biophys Acta 341:497–504, 1974.
Ohnishi M, Suganuma T, Fujimori H, Hiromi K: Role of tryptophan residue of bacterial liquefying α-amylase*1 and Taka-amylase A*2 in enzymatic hydrolysis of linear substrates. J Biochem 74:1271–1273, 1973.
Kochhar S, Batra R, Dua RD: Purification and characterization of thermostable α-amylase from Bacillus amyloliquefaciens-159 Enzyme Microbiol Tech: Communicated, 1984.
Bernfeld, P: Amylases, α and β. In: Colowick SP, Kaplan NO (eds), Methods in Enzymology, Vol 1. Academic Press, New York, 1955, pp 149–158.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurements with folin phenol reagent. J Biol Chem 193:265–275, 1952.
Roosemont JL: Reaction of histidine residues in proteins with diethylpyrocarbonate; Differential molar absorptivities and reactivities. Anal Biochem 88:314–320, 1978.
Ovadi J, Libor S, Elodi P: Spectrophotometric determination of histidine in proteins with diethylpyrocarbonate. Acta Biochim Biophys Acad Sci Hung 2:455–458, 1967.
Vesterberg O: Isoelectric focussing of proteins in polyacrylamide gels. Biochim Biophys Acta 257:11–19, 1972.
Rippa M, Poontremoll S: Evidence for a critical histidine residue in 6-phosphogluconate dehydrogenase from Candida utilis. Biochemistry 7:1514–1518, 1968.
Scrutton M, Utter M: Pyruvate carboxylase V: Interaction of the enzyme with Adenosine Triphosphate. J Biol Chem 240:3714–3723, 1965.
Wells MA: Effects of chemical modification on the activity of Cratalus adamanteus phospholipase A2. Evidence for an essential amino group. Biochemistry 12:1086–1093, 1973.
Melchior WB Jr, Fahrney D: Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with α-chymotripsin, pepsin and pancreatic ribonuclease at pH 4.0. Biochemistry 9:251–258, 1970.
Hegyi G, Premeez G, Sain B, Muhlard A: Selective carbethoxylation of the histidine residues of actin by diethylpyrocarbonate. Eur J Biochem 44:7–12, 1974.
Miles EW: Carbethoxylation of histidine by diethylpyrocarbonate (DEP). In: Hiris CHW, Timasheff SN (eds), Methods in Enzymology, Vol 47, Part E. Academic Press, New York, 1977, pp 431–442.
Muhlrad A, Hegyi G, Toth G: Effect of diethylpyrocarbonate on proteins (I): reaction of diethylpyrocarbonate with amino acids. Acta Biochim Biophys Acad Sci Hung 2:19–29, 1967.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dua, R.D., Kochhar, S. Active site studies on Bacillus amyloliquefaciens α-amylase (I). Mol Cell Biochem 66, 13–20 (1985). https://doi.org/10.1007/BF00231818
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00231818