Summary
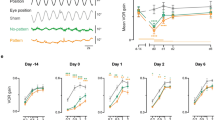
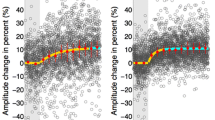
When a normal human subject is briefly turned in total darkness while trying to “look” at a spatially fixed target, the vestibulo-ocular reflex (VOR) produces slow-phase compensatory eye movements tending to hold the eyes on target. However, slow-phase compensation per se is generally inadequate in these circumstances. Nevertheless it has recently been found, that even in the dark, this inadequacy tends to be corrected by supplementary saccades usually acting in the compensatory direction. The present study further investigates this phenomenon by measuring the respective contributions of saccadic, slow-phase and overall net compensation in 9 subjects tested before and after 30% adaptive attenuation of VOR slow-phase gain. In each test series, subjects attempted to stabilize their gaze on a previously seen target during each of 40 brief (≈0.5 s) whole body rotations (40°/s, 20° amp) conducted in complete darkness. The adaptive experience comprised 2 h of full-field visual suppression of the VOR during sinusoidal rotation of subject and surround at 1/6 Hz and 40°/s velocity amplitude. Before adaptation, the cumulative slow-phase and cumulative saccadic components produced on average 78% and 14% respectively of the ideal (100%) compensation, thus yielding an overall net compensation which was 92% of the desired value. After adaptation, the corresponding values in the same population were 53%, 18% and 71% respectively. Thus after adaptation, the combined saccadic-slow-phase response brought the final gaze position to a point in space that was systematically shifted in the direction of head rotation (i.e. undercompensation). Subjects re-exposed to 30 min of normal visual-vestibular interaction displayed a variety of recovery patterns using different combinations of slow and saccadic eye movements. However, there was a consistent “synergistic” tendency for saccadic eye movements to improve slow-phase performance, regardless of the subject's adaptive state. In one subject, compensatory saccadic eye movements corrected a consistent directional asymmetry in the slow-phase response. It is suggested that a conscious vestibular percept of self-rotation might underlie the combined saccadic-slow-phase response, and that the net under performance after adaptation might reflect attenuation of this percept relative to the actual rotational stimulus.
Similar content being viewed by others
References
Baloh RW, Lyerly K, Yee YD, Honrubia V (1984) Voluntary control of the human vestibulo-ocular reflex. Acta Otolaryngol (Stockh) 97:1–6
Barr CC, Schultheis LW, Robinson DA (1976) Voluntary, non-visual control of the human vestibulo-ocular reflex. Acta Otolaryngol (Stockh) 81:365–375
Berthoz A (1988) The role of gaze in compensation of vestibular disfunction: the gaze substitution hypothesis. Progr Brain Res 76:411–420
Berthoz A, Melvill Jones G (eds) (1985) Adaptive mechanisms in gaze control. Elsevier Biomed Press, Amsterdam
Bloomberg J, Melvill Jones G, Segal B (1986a) Quick and slow phase interaction following adaptive attenuation of the human vestibulo-ocular reflex. Can J Physiol Pharmacol 64: A3
Bloomberg J, Melvill Jones G, Segal B (1986b) Does the quick phase supplement of the goal-directed vestibulo-ocular reflex (VOR) reflect the adaptive state of a modifiable internal spatial map? Soc Neurosci Abstr 12, Part 2, p 1090
Bloomberg J, Melvill Jones G, Segal B (1991) Adaptive modification of vestibularly perceived rotation. Exp Brain Res 84:47–56
Furst EJ, Goldberg J, Jenkins HA (1987) Voluntary modification of the rotatory induced vestibulo-ocular reflex by fixating imaginary targets. Acta Otolaryngol (Stockh) 103:232–240
Gauthier GM, Robinson DA (1975) Adaptation of the human vestibulo-ocular reflex to magnifying lenses. Brain Res 92:331–335
Gonshor A, Melvill Jones G (1971) Plasticity in the adult human vestibulo-ocular reflex arc. Proc Can Fed Biol Soc 14:11
Gonshor A, Melvill Jones G (1976a) Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol (Lond) 256:361–379
Gonshor A, Melvill Jones G (1976b) Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol (Lond) 256:381–414
Guedry FE (1974) Psychophysics of vestibular sensation. In: Kornhuber HH (ed) Handbook of sensory physiology. Vol V1/2. Springer Berlin Heidelberg New York, pp 1–154
Guedry Jr FE, Stockwell CW, Gilson RD (1971a) Comparison of subjective responses to semicircular canal stimulation produced by rotation about different axes. Acta Otolaryngol (Stockh) 72:101–106
Guedry Jr FE, Stockwell CW, Norman JW, Owens GG (1971b) Use of triangular waveforms of angular velocity in the study of vestibular function. Acta Otolaryngol (Stockh) 71:439–448
Hunter IW, Kearney RE (1984) Nexus: a computer language for physiological systems and signal analysis. Comput Biol Med 14:385–401
Istl-Lenz Y, Hyden D, Schwartz DWF (1985) Response of the human vestibulo-ocular reflex following long-term 2 x magnified visual input. Exp Brain Res 57:448–455
Ito M (1982) Cerebellar control of the vestibulo-ocular reflex: around the flocculus hypothesis. Ann Rev Neurosci 5:275–296
Ito M, Shiida T, Yagi N, Yamamoto M (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res 65:170–174
Ito M, Jastreboff PJ, Miyashita Y (1979) Adaptive modification of the rabbit's horizontal vestibulo-ocular reflex during sustained vestibular and optokinetic stimulation. Exp Brain Res 37:17–30
Kasai T, Zee DS (1978) Eye-head coordination in labyrinthine-defective human beings. Brain Res 144:123–141
Krogh E (1979) The corneofundal potential and the electrooculogram: aspects of normal physiology and variability. Acta Ophthalmol Suppl (Copenh) 140:135–141
McKinley PA, Peterson BW (1985) Voluntary modulation of the vestibulo-ocular reflex in humans and its relation to smooth pursuit. Exp Brain Res 60:454–464
Melvill Jones G (1977) Plasticity in the adult vestibulo-ocular reflex arc. Phil Trans R Soc Lond [Biol] 278:319–334
Melvill Jones G, Gonshor A (1982) Oculomotor response to rapid head oscillation (0.5–5.0 Hz) after prolonged adaptation to vision-reversal: “simple” and “complex” effects. Exp Brain Res 45:45–58
Melvill Jones G, Berthoz A, Segal B (1984) Adaptive modification of the vestibulo-ocular reflex by mental effort in darkness. Exp Brain Res 56:149–153
Melvill Jones G, Segal B, Katsarkas A, Bloomberg J (1988) Experiments on vestibular adaptation and its clinical significance. In: Barber HO, Sharpe JA (eds) Vestibular disorders. Year Book Medical Publ, Chicago, pp 3–23
Miles FA, Fuller JH (1974) Adaptive plasticity in the vestibulo-ocular responses of the rhesus monkey. Brain Res 80:512–516
Miles FA, Eighmy BB (1980) Long-term adaptive changes in primate vestibulo-ocular reflex. I. Behavioural observations. J Neurophysiol 43:1406–1425
Miles FA, Lisberger SG (1981) Plasticity in the vestibulo-ocular reflex: a new hypothesis. Ann Rev Neurosci 4:273–299
Robinson DA (1976) Adaptive gain control of vestibulo-ocular reflex by the cerebellum. J Neurophysiol 39:954–969
Robinson DA (1981) Control of eye movements. In: Handbook of physiology: the nervous system, Sect 1. Am Physiol Soc 22(2):1275–1320
Segal BN, Katsarkas A (1988a) Goal-directed vestibulo-ocular function in man: gaze stabilization by slow-phase and saccadic eye movements. Exp Brain Res 70:26–32
Segal BN, Katsarkas A (1988b) Long-term deficits of goal-directed vestibulo-ocular function following total unilateral loss of peripheral vestibular function. Acta Otolaryngol (Stockh) 106:102–110
Wilson VJ, Melvill Jones G (1979) Mammalian vestibular physiology. Plenum Press, New York
Young LR (1984) Perception of the body in space. In: Darian-Smith I (ed) Handbook of physiology — the nervous system III. Am Physiol Assoc. Waverly Press, Baltimore MD, pp1023–1066
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bloomberg, J., Jones, G.M. & Segal, B. Adaptive plasticity in the gaze stabilizing synergy of slow and saccadic eye movements. Exp Brain Res 84, 35–46 (1991). https://doi.org/10.1007/BF00231760
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231760