Summary
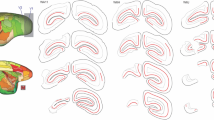
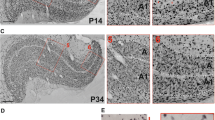
The superior colliculus was bilaterally or unilaterally ablated at different early postnatal ages in rats. When adult, each rat received a unilateral eye injection of Horesradish peroxidase to reveal the crossed and uncrossed retinal terminal fields within the dorsal lateral geniculate nucleus. Collicular ablation in the first seven days after birth, but not thereafter, produced a small hole or vacancy within the contralateral retinal terminal field which was occupied by an aberrant ipsilateral retinal terminal field. These rearrangements in the retino-geniculate projections occurred in the caudal quarter of the nucleus dorso-laterally just beneath the optic tract, solely ipsilateral to the ablated colliculus. Possible causes of the formation of these rearrangements are discussed, and similarities with other aberrant retinal projections following early damage to the visual system are considered.
Similar content being viewed by others
Abbreviations
- dLGN:
-
Dorsal geniculate nucleus
- DTN:
-
Dorsal terminal nucleus of the accessory optic tract
- HRP:
-
Horseradish peroxidase
- LP:
-
Latero-posterior nucleus
- NOT:
-
Nucleus of the optic tract
- OT:
-
Optic tract
- PO:
-
Olivary pretectal nucleus
- PP:
-
Posterior pretectal nucleus
- SC:
-
Superior colliculus
- TMB:
-
Tetramethyl benzidine
References
Adams AD, Forrester JM (1968) The projection of the rat's visual field on the cerebral cortex. Q J Exp Physiol 53: 327–336
Baisinger J, Lund RD, Miller B (1977) Aberrant retinothalamic projections resulting from unilateral tectal lesions made in fetal and neonatal rats. Exp Neurol 54: 369–382
Cowey A, Henken DB, Perry VH (1982) Effects on visual acuity of neonatal or adult tectal ablation in rats. Exp Brain Res 48: 149–152
Cowey A, Perry VH (1979) The projection of the temporal retina in rats, studied by retrograde transport of horseradish peroxidase. Exp Brain Res 35: 457–464
Grain BJ, Hall WC (1980a) The organization of the lateral posterior nucleus of the golden hamster after neonatal superior colliculus lesions. J Comp Neurol 193: 383–401
Grain BJ, Hall WC (1980b) The organization of afferents to the lateral posterior nucleus in the golden hamster after different combinations of neonatal lesions. J Comp Neurol 193: 403–412
Dean P (1981) Visual acuity in hooded rats: effects of superior collicular or posterior neocortical lesions. Brain Res 156: 17–31
Frost DO, Schneider GE (1979) Plasticity of retinofugal projections after partial lesions of the retina in newborn Syrian hamsters. J Comp Neurol 185: 517–567
Fukuda Y (1977) A three group classification of rat retinal ganglion cells: histological and physiological studies. Brain Res 119: 327–344
Giolli RA, Creel DJ (1974) Inheritance and variability of the organization of the retinogeniculate projections in pigmented and albino rats. Brain Res 78: 335–339
Jeffery G (1984) Retinal ganglion cell death and terminal field retraction in the developing rodent visual system. Develop Brain Res 13: 81–96
Lieberman AR (1974) Some factors affecting retrograde neuronal responses to axonal lesions. In: Bellairs R, Gray EG (eds) Essays on the nervous system. Clarendon Press, Oxford, Chap 4
Linden R, Cowey A, Perry VH (1983) Tectal ablation at different ages in developing rats has different effects on retinal ganglion cell density but not on visual acuity. Exp Brain Res 51: 368–376
Linden R, Perry VH (1982) Ganglion cell death within the developing retina: a regulatory role for retinal dendrites? Neuroscience 7: 2813–2827
Linden R, Perry VH (1983) Massive retinotectal projection in rats. Brain Res 272: 145–149
Lund RD (1965) Uncrossed visual pathways of hooded and albino rats. Science 149: 1506–1507
Lund RD, Cunningham TJ, Lund JS (1973) Modified optic projections after unilateral eye removal in young rats. Brain Behav Evol 8: 51–72
Lund RD, Land PW, Boles J (1980) Normal and abnormal uncrossed retinotectal pathways in rats: an HRP study in adults. J Comp Neurol 189: 711–720
Lund RD, Lund JS (1976) Plasticity in the developing visual system: the effects of retinal lesions made in young rats. J Comp Neurol 169: 133–154
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Montero VM, Brugge JF, Beitel RE (1968) Relation of the visual field to the lateral geniculate body of the albino rat. J Neurophysiol 31: 221–236
Perry VH (1980) A tectocortical visual pathway in the rat. Neuroscience 5: 915–927
Perry VH, Cowey A (1979a) The effects of unilateral cortical and tectal lesions on retinal ganglion cells in rats. Exp Brain Res 35: 85–95
Perry VH, Cowey A (1979b) Changes in the retino-fugal pathways following cortical and tectal lesions in neonatal and adult rats. Exp Brain Res 35: 97–108
Perry VH, Cowey A (1982) A sensitive period for ganglion cell degeneration and the formation of aberrant retino-fugal connections following tectal lesions in rats. Neuroscience 7: 583–594
Reese BE (1984) The projection from the superior colliculus to the dorsal lateral geniculate nucleus in the rat. Brain Res 305: 162–168
Reese BE, Cowey AC (1983) Projection lines and the ipsilateral retino-geniculate pathway in the hooded rat. Neuroscience 10: 1233–1247
Reese BE, Jeffery G (1983) Crossed and uncrossed visual topography in dorsal lateral geniculate nucleus of the pigmented rat. J Neurophysiol 49: 877–885
Scalia R, Arango V (1979) Topographic organization of the projections of the retina to the pretectal region in the rat. J Comp Neurol 186: 271–292
Schneider GE (1973) Early lesions of superior colliculus: factors affecting the formation of abnormal retinal projections. Brain Behav Evol 8: 73–109
Schober W, Gruschka H (1977) Die Ganglienzellen der Retina der Albinoratte: eine qualitative und quantitative Studie. Z Mikrosk-Anat Forsch 91: 397–414
Siminoff R, Schwassmann HO, Kruger L (1966) An electrophysiological study of the visual projection to the superior colliculus of the rat. J Comp Neurol 127: 435–444
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reese, B.E., Cowey, A. Rearrangements in the retino-geniculate projections of rats following ablation of the superior colliculus in infancy. Exp Brain Res 59, 372–381 (1985). https://doi.org/10.1007/BF00230917
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230917