Abstract
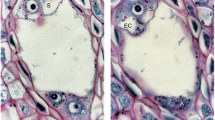
Nuclear DNA amounts (C values) were measured in Feulgen-stained sections of anthers and ovules of sexual plant B-2s (genotype aaaa) and aposporous cultivar Higgins (genotype AAaa) of buffelgrass (Pennisetum ciliare). The mass of the unreplicated nuclear genome of a gamete equals 1C DNA. In both lines, pollen mother cell nuclei were 4C before leptotene; anther wall, dyad, 1-nucleate pollen, and generative cell nuclei were 2C; microspore tetrad, enlarging microspore, and sperm nuclei were 1C. The tapetum persisted as uninucleate cells with 4C DNA. Archespores (2-4C) of both lines initiated meiosis to form megaspore tetrad nuclei with 1-2C DNA. In B-2s, chalazal megaspores (2-4C) formed reduced 8-nucleate Polygonum type embryo sacs, and sacs at 2- and 4-nucleate stages showed distributions with peaks near C1 and C2, corresponding to G1 and G2 cell cycle phases; this is characteristic of active mitosis. Nuclei of 8-nucleate sacs and of eggs and polars were 1C, indicating chromosomes were not duplicated before fertilization. Antipodal nuclei had levels from 1 to 36C, possibly due to polyteny or endopolyploidy. In Higgins, aposporous initials and 2-nucleate embryo sacs showed bimodal distributions of 2n nuclei with peaks at 2C and 4C DNA. Nuclei of newly formed 4-nucleate Panicum type aposporous sacs and of polars were 2C; aposporous eggs stained too faintly for reliable measurement.
Similar content being viewed by others
References
Asker SE, Jerling L (1992) Apomixis in plants. CRC Press, Boca Raton, Fla
Bashaw EC (1962) Apomixis and sexuality in buffelgrass. Crop Sci 2:412–115
Bashaw EC (1968) Registration of Higgins buffelgrass. Crop Sci 8:397–398
Bashaw EC, Hanna WW (1990) Apomictic reproduction. In: Chapman GP (ed) Reproductive versatility in the grasses. Cambridge University Press, Cambridge, UK, pp 100–130
Bennett MD (1976) The cell in sporogenesis and spore development. In: Yeoman MM (ed) Cell division in higher plants. Academic Press, New York, pp 161–198
Bennett MD (1985) Intraspecific variation in DNA amount and the nucleotypic dimension in plant genetics. In: Freeling M (ed) Plant genetics. Liss, New York, pp 283–302
Bennett MD, Finch RA, Smith JB, Rao MK (1973) The time and duration of female meiosis in wheat, rye and barley. Proc R Soc Lond B 183:301–319
Bennett MD, Smith JB (1976) Nuclear DNA amounts in angiosperms. Philos Trans R Soc Lond B 274:227–274
Bennett MD, Smith JB, Heslop-Harrison JS (1982) Nuclear DNA amounts in angiosperms. Proc R Soc Lond B 216:179–199
Bhaskaran S, Swaminathan MS (1959) Stage of deoxyribonucleic acid synthesis during mitosis and meiosis. Curr Sci 8:335–336
Bryant TR, Howard KL (1969) Meiosis in the oomycetes. I. A microspectrophotometric analysis of nuclear deoxyribonucleic acid in Saprolegnia terrestris. Am J Bot 56:1075–1083
D'Amato F, Devreux M, Scarascia Mugnozza GT (1965) The DNA content of the nuclei of the pollen grains in tobacco and barley. Caryologia 18:377–382
Davis GL (1966) Systematic embryology of the angiosperms. Wiley, New York
Fisher WD, Bashaw EC, Holt EC (1954) Evidence for apomixis in Pennisetum ciliare and Cenchrus setigerus. Agron J 46:401–404
Gounaris EK, Sherwood RT, Gounaris I, Hamilton RH, Gustine DL (1991) Inorganic salts modify embryo sac development in sexual and aposporous Cenchrus ciliaris Sex Plant Reprod 4:188–192
Hu SY (1964) Morphological and cytological observations on the process of fertilization of the wheat. Sci Sin 13:925–936
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Kaltsikes PJ (1973) Early seed development in hexaploid triticale. Can J Bot 51:2291–2300
Kapil RN, Bhatnagar AK (1981) Ultrastructure and biology of female gametophyte in flowering plants. Int Rev Cytol 70:291–341
Le Coq C (1972) La megasporogenese chez l'Iris pseudacorus L. II. Etude cytologique quantitative. Rev Cytol Biol Veg 35:303–330
Moses MJ, Tayler JH (1955) Deoxypentose nucleic acid synthesis during microsporogenesis in Tradescantia. Exp Cell Res 9:474–488
Nagl W (1976) The polytenic antipodal cells in Scilla bifolia: DNA replication pattern and possibility of nucleolar DNA amplification. Cytobiology 14:165–170
Noeske K (1971) Discrepancies between cytophotometric Feulgen values and deoxyribosenucleic acid content. J Histochem Cytochem 19:169–174
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 475–518
Pritchard HN (1964) A cytochemical study of embryo sac development in Stellaria media. Am J Bot 51:371–378
Rutishauser A (1969) Embryologie und Fortpflanzungsbiologie der Angiospermen. Spring, Vienna New York
Sherwood RT, Berg CG, Young BA (1994) Inheritance of apospory in buffelgrass. Crop Sci 34:1490–1494
Snyder LA, Hernandez AR, Warmke HE (1955) The mechanism of apomixis in Pennisetum ciliare. Bot Gaz 116:209–221
Sukesh-Kumar CP, Shah CK (1986) Histochemistry of apomictic (Ranunculus auricomus L) and meiotic (Ranunculus cassubicifolius W Koch) embryo sacs. Acta Bot Indica 14:226–229
Swift H (1950) The constancy of deoxyribose nucleic acid in plant nuclei. Proc Natl Acad Sci USA 36:643–654
Taylor JH (1958) Incorporation of phosphorus-32 into nucleic acids and proteins during microgametogenesis of Tulbaghia. Am J Bot 45:123–131
Taylor JH, McMaster RD (1954) Autoradiographic and microphotometric studies of deoxyribose nucleic acid during microgametogenesis in Lilium longiflorum. Chromosoma 6:489–521
Warmke HE (1954) Apomixis in Panicum maximum Am J Bot 41:5–11
Weir CE, Dale HM (1960) A developmental study of wild rice, Zizania aquatica L. Can J Bot 38:719–739
Woodard JW (1956) DNA in gametogenesis and embryogeny in Tradescantia. J Biophys Biochem Cytol 2:765–776
Woodcock CLF, Bell PR (1968) The distribution of deoxyribonucleic acid in the female gametophyte of Myosurus minimus. Histochemie 12:289–301
Xi X-Y, DeMason DA (1984) Relationship between male and female gametophyte development in rye. Am J Bot 71:1067–1079
Author information
Authors and Affiliations
Additional information
Names of products are included for the benefit of the reader and do not imply endorsement or preferential treatment by USDA
Rights and permissions
About this article
Cite this article
Sherwood, R.T. Nuclear DNA amount during sporogenesis and gametogenesis in sexual and aposporous buffelgrass. Sexual Plant Reprod 8, 85–90 (1995). https://doi.org/10.1007/BF00230893
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230893