Abstract
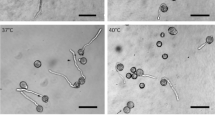
The germination and growth of pollen grains of Nicotiana tabacum and N. alata with the anti-microtubule drug oryzalin retarded significantly the movement of the vegetative nucleus (VN) and the generative cell (GC) from the grain to the tube apex but had no effect on pollen tube elongation. In N. tabacum, only 11% and 48% of the pollen tubes treated with oryzalin for 6 h and 12 h, respectively, had the VN and GC in the tube mainly in its middle part. In corresponding control materials, 79% and 99% of pollen tubes contained the VN and GC close to the apex. Indirect immunofluorescence microscopy and related studies of the tubes grown in the presence of oryzalin revealed complete absence of microtubules (MTs) but apparently intact microfilaments (MFs). These results suggested that the movement of VN and GC from the grain into the tube is possible when no MTs but only MFs are present, but the movement is then slow. In control tubes, the parallel orientation of MT bundles and extensions of VN were interpreted to represent the structural organization needed for the MT-dependent movement of VN.
Similar content being viewed by others
References
Åström H (1992) Acetylated α-tubulin in the pollen tube microtubules. Cell Biol Int Rep 16:871–881
Åström H, Virtanen I, Raudaskoski M (1991) Cold-stability in the pollen tube cytoskeleton. Protoplasma 160:99–107
Cai G, Bartalesi A, Del Casino C, Moscatelli A, Tiezzi A, Cresti M (1993) The kinesin-immunoreactive homologue from Nicotiana tabacum pollen tubes: biochemical properties and subcellular localization. Planta 191:496–506
Cresti M, Murgia M, Theunis CH (1990) Microtubule organization in sperm cells in the pollen tubes of Brassica oleracea L. Protoplasma 154:151–156
Del Casino C, Li Y, Moscatelli A, Scali M, Tiezzi A, Cresti M (1993) Distribution of microtubules during the growth of tobacco pollen tubes. Biol Cell 79:125–132
Franke WW, Herth W, VanDer Woude WJ, Morré DJ (1972) Tubular and filamentous structures in pollen tubes: possible involvement as guide elements in protoplasmic streaming and vectorial migration of secretory vesicles. Planta 105:317–341
Heslop-Harrison J (1988) The pollen tube: motility and the cytoskeleton. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin Heidelberg New York, pp 195–203
Heslop-Harrison J, Heslop-Harrison Y (1989a) Conformation and movement of the vegetative nucleus of the angiosperm pollen tube: association with the actin cytoskeleton. J Cell Sci 93:299–308
Heslop-Harrison J, Heslop-Harrison Y (1989b) Myosin associated with the surfaces of organelles, vegetative nuclei and generative cells in angiosperm pollen grains and tubes. J Cell Sci 94:319–325
Heslop-Harrison J, Heslop-Harrison Y (1992) Intracellular motility, the actin cytoskeleton and germinability in the pollen of wheat (Triticum aestivum L.). Sex Plant Reprod 5:247–255
Heslop-Harrison J, Heslop-Harrison Y, Cresti M, Tiezzi A, Ciampolini F (1986) Actin during pollen germination. J Cell Sci 86:1–8
Heslop-Harrison J, Heslop-Harrison Y, Cresti M, Tiezzi A, Moscatelli A (1988) Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J Cell Sci 91:49–60
Kajstura J, Bereiter-Hahn J (1993) Disruption of microtubules induces formation of actin fibrils in density-inhibited 3T3 cells. Cell Biol Int 17:1023–1031
Kohno T, Ishikawa R, Nagata T, Kohama K, Shimmen T (1992) Partial purification of myosin from lily pollen tubes by monitoring with in vitro motility assay. Protoplasma 170:77–85
Lancelle SA, Hepler PK (1991) Association of actin with cortical microtubules revealed by immunogold localization in Nicotiana pollen tubes. Protoplasma 165:167–172
Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5:1303–1314
Morejohn LC (1991) The molecular pharmacology of plant tubulin and microtubules. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, New York, pp 29–43
Morejohn LC, Bureau TE, Molè-Bajer J, Bajer AS, Fosket DE (1987) Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264
Moscatelli A, Tiezzi A, Vignani R, Cai G, Bartalesi A, Cresti M (1988) Presence of kinesin in tobacco pollen tube. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin Heidelberg New York, pp 205–209
Ozawa T, Takeshita M, Negishi O, Imagawa H (1993) Pollen tube growth promoters from the style of Rhododendron mucronatum G. Don. Biosci Biotech Biochem 57:2122–2126
Palevitz BA (1993) Relationship between the generative cell and vegetative nucleus in pollen tubes of Nicotiana tabacum. Sex Plant Reprod 6:1–10
Palevitz BA, Tiezzi A (1992) Organization, composition, and function of the generative cell and sperm cytoskeleton. Int Rev Cytol 140:149–185
Pierson ES, Cresti M (1992) Cytoskeleton and cytoplasmic organization of pollen and pollen tubes. Int Rev Cytol 140:73–125
Pierson ES, Kengen HMP, Derksen J (1989) Microtubules and actin filaments co-localize in pollen tubes of Nicotiana tabacum L. and Lilium longiflorum Thunb. Protoplasma 150:75–77
Polya GM, Micucci V, Rae AL, Harris PJ, Clarke AE (1986) Ca2+ -dependent protein phosphorylation in germinated pollen of Nicotiana alata, an ornamental tobacco. Physiol Plant 67:151–157
Raudaskoski M, Åström H, Perttilä K, Virtanen I, Louhelainen J (1987) Role of the microtubule cytoskeleton in pollen tubes: an immunocytochemical and ultrastructural approach. Biol Cell 61:177–188
Read SM, Clarke AE, Bacic A (1993) Requirements for division of the generative nucleus in cultured pollen tubes of Nicotiana. Protoplasma 174:101–115
Shimmen T, Yoshida S (1993) Analysis of temperature dependence of cytoplasmic streaming using tonoplast-free cells of Characeae. Protoplasma 176:174–177
Strachan SD, Hess FD (1983) The biochemical mechanism of action of the dinitroaniline herbicide oryzalin. Pestic Biochem Physiol 20:141–150
Tang X, Hepler PK, Scordilis SP (1989) Immunochemical and immunocytochemical identification of a myosin heavy chain polypeptide in Nicotiana pollen tubes. J Cell Sci 92:569–574
Tiezzi A (1991) The pollen tube cytoskeleton. Electron Microsc Rev 4:205–219
Tiezzi A, Moscatelli A, Cai G, Bartalesi A, Cresti M (1992) An immunoreactive homolog of mammalian kinesin in Nicotiana tabacum pollen tubes. Cell Motil Cytoskel 21:132–137
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol 105:387–395
Vale RD, Goldstein LSB (1990) One motor, many tails: an expanding repertoire of force-generating enzymes. Cell 60:883–885
Yokota E, Shimmen T (1994) Isolation and characterization of plant myosin from pollen tubes of lily. Protoplasma 177:153–162
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Åström, H., Sorri, O. & Raudaskoski, M. Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sexual Plant Reprod 8, 61–69 (1995). https://doi.org/10.1007/BF00230890
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230890