Abstract
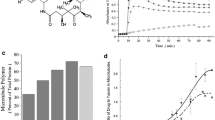
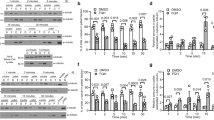
The antineoplasic drug estramustine is an adduct of estradiol and nor-nitrogen mustard. It has been shown that this drug interferes with microtubule assembly, an effect mediated by estramustine interaction with microtubule-associated proteins (MAPS). In the present report we demonstrate that estramustine and the phosphorylated derivative of the drug, estramustine-phosphate, inhibit the secretion of interleukin-3 by WEHI-3B cells. These studies also show that the estramustine derivative specifically interacts with a MAPs component found in these cells, which exhibited characteristics ressembling those of tau protein isoforms. Western blots using a unique monoclonal antibody MTB6.22 that recognizes microtubule-binding domains on MAPs, indicated that this WEHI protein factor contained the antigenic determinant that are functionally significant for microtubule assembly. ELISA assays using this antibody, also showed a decrease in the levels of the immunoreactive protein in WEHI cells after treatment with EMP. Interestingly, it has been recently described that the action of estramustine-phosphate is mediated by a direct interaction with MAP-binding sites on the microtubule surface, which are recognized by the site-specific monoclonal antibody. These findings together with immuno-precipitation experiments using anti-interleukin-3 antibodies and the inhibitory effect of the estramustine derivative on WEHI secretion process suggest that this anti-mitotic agent may block IL-3 secretion by a mechanism involving its interaction with a ‘tau-like’ MAPs component present in these cells.
Similar content being viewed by others
References
Bershadsky A, Vasiliev J: In Citoskeleton. Plenum Press, 1988
Dainiak N, Riordan MA, Strauss PR, Feldman L, Kreczko S: Contractile proteins participate in release of erythroid growth regulators from mononuclear cells. Blood 72: 165–171, 1988
Liotta LA, Sterg PS, Sletler-Stevenson WG: Cancer methastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 64: 327–336, 1991
Liotta LA, Tryggvanson K, Garbisa S, Hart I, Foltz CM, Shafie S: Methastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature (London) 284: 67–68, 1980
Heldin CH, Wastermark B: Growth factors as transforming proteins. Eur J Biochem 184: 487–496, 1989
Maccioni RB, Arechaga J: The cytoskeleton in cell differentiation and development. IRL Oxford University Press. 1987, pp 395
Shiomura Y, Hirokawa N: Localization of MAP-lA and MAP-2 on neuronal microtubules in situ revealed with double label immunoelectron microscopy. J Cell Biol 104: 1575–1578, 1987
Schpetner HS, Paschal BM, Vallee RB: Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP-1C). J Cell Biol 107: 1001–1009, 1988
Stearns M, Wang M, Sousa O: Evidence that estramustine binds MAP-1A to inhibits type IV collagenase secretion. J Cell Sci 98: 55–62, 1991
Wang M, Tew KD, Stearns M: Immunofluorescence studies of the anti-microtubule effects of anticancer drug estramustine. Anticancer Res 7: 1165–1172, 1987
Wallin M, Deinum J, Friden B: Interactions of estramustine-phosphate with microtubule-associated proteins. FEBS Lett 179: 289–293, 1985
Friden B, Rutberg M, Deinum J, Wallin M: The effects of estramustine derivatives on microtubule assembly in vitro depends on the charge of the substituents. Biochem Pharmacol 42: 997–1006, 1991
Metcalf D, Moore MAS, Warner NL: Colony formation in vitro by myelomonocytic leukemic cells. J Natl Cancer Inst 43: 983–1001, 1969
Metcalf D: Haematopoietic colonies in vitro: Cloning of normal and leukaemic cells. Springer Verlag, Berlin and Heidelberg, 1977, pp 227
Dexter TM, Garland J, Scott D, Scolnick E, Metcalf D: Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med 152: 1036–1039, 1979
Seeds NW, Maccioni RB: Characterization of a microtubule assembly promoting-factor. J Cell Biol 76: 54–556, 1978
Vera JC, Rivas C, Maccioni RB: Heat-stable microtubule proteins MAP-1 binds to microtubules and induces microtubule assembly. FEBS Lett 232: 159–162, 1989
Padilla R, Maccioni RB, Avila J: Calmodulin binds to a tubulin binding site of the microtubule-associated protein tau. Mol Cell Biochem 97: 35–41, 1990
Laemmli U: Cleavage of the structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227: 680–685, 1970
Morrisey JM: Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal Biochem 117: 307–320, 1981
Rivas C, Vera JC, Maccioni RB: Anti-idiotypic antibodies reacting with MAPS in the sera of rabbits immunized with synthetic peptides from tubulin's regulatory domain. Proc Natl Acad Sci USA 85: 6092–6096, 1988
Farías F, Vial C, Maccioni RB: Specific macromolecular interactions between tau and the microtubule system. Mol Cell Biochem 112: 81–88, 1992
Vera JC, Rivas C, Maccioni RB: Antibodies to synthetic peptides from the tubulin regulatory domain interact with tubulin and microtubules. Proc Natl Acad Sci USA 85: 6763–6767, 1988
Cann J, York E, Stewart JM, Vera JC, Maccioni RB: Small-zone gel chromatography of interacting systems: Theoretical and experimental evaluation of elution profiles for kinetically-controlled macromolecule-ligand reactions. Anal Biochem 175: 462–473, 1988
Sheridan RV, Spicher LA, Tew KD: The effects of estramustine on metaphase and anaphase in DU-145 prostatic carcinoma cells. Eur J Cell Biol 54: 268–276, 1991
Moraga D, Rivas-Berrios A, Farfas G, Wallin M, Maccioni RB: Estramustine-phosphate binds to a tubulin binding domain on microtubule associated proteins MAP-2 and tau. Biochem Biophys Acta 1121: 97–103, 1992
Friden B, Wallin M, Deinum J, Prasad V, Ludueña R: Effects of estramustine-phosphate on the assembly of trypsin-treated microtubules and microtubules reconstituted from purified tubulin with either tau, MAP-2 or the tubulin binding fragment of MAP-2. Arch Biochem Biophys 257: 123–130, 1987
Stearns M, Wang M, Tew K, Binder L: Estramustine binds a MAP-1 like protein to inhibit microtubule assembly in vitro and disrupt microtubule organization in DU-145 cells. J Cell Biol 107: 2647–2656, 1988
Lee YL, Wolff J: Calmodulin binds to both microtubule-associated protein 2 and tau proteins. J Biol Chem 259: 1226–1230, 1984
Drubin D, Kobayashi S, Kirschner MW: Association of tau proteins with microtubules in living cells. Ann N.Y. Acad Sci 466: 257–268, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martínez, J., Santibáñez, J.F., Vial, C. et al. The antineoplasic agent estramustine and the derivative estramustine-phosphate inhibit secretion of interleukin-3 in leukemic cells. Possible roles of MAPs. Mol Cell Biochem 117, 165–173 (1992). https://doi.org/10.1007/BF00230756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230756