Abstract
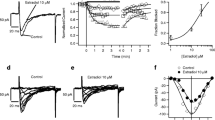
The effect of incubating αT3-1 cells with phorbol 12,13-dibutyrate (PDBu) on the protein kinase C (PKC) isoform content (predominantly α, ɛ and ζ isoforms) was assessed by immunoblotting, enzyme activity assay and [3H]PDBu binding. After exposure to PDBu for 17 h the immunoreactivity detected for both PKC α and PKC ɛ had disappeared from cytosol and had increased slightly in membranes. Immunoreactivity for PKC ζ was present as two bands in cytosol; after PDBu treatment both bands decreased in intensity, the higher molecular weight band more than the lower. The lower molecular weight band corresponded with a component of constitutive PKC activity eluting from DEAE cellulose that was defined by inhibition of basal activity with GF 109203X or H7. Investigation of very short treatment times with PDBu using binding, immunoblot and activity measurements (in the presence/absence of Ca2+) indicated that translocation of PKC α and ɛ was very rapid — detectable by 10 sec, maximal within minutes. Reduction of these isoforms in membranes took much longer, and was not apparent up to 150 min. The immunoblot data for PKC ζ in cytosol showed no detectable effect of PDBu treatment on the low molecular weight band up to 150 min although it was reduced at 17 h. Translocation of the upper band was detectable at 10 sec but this band may have resulted from cross-reaction with other PKC isoforms. The constitutive activity and low molecular weight (‘authentic’) PKC ζ immunoreactivity were partially affected after long exposure only, suggesting an action of PDBu on PKC ζ secondary to activation of the other PKC isoforms. An endogenous receptor agonist, luteinising hormone-releasing hormone (LHRH), was also used to assess by immunoblotting, translocation of the PKC isoforms. Although all the isoforms did translocate from cytosol to membrane fractions, they did so with distinctly different time courses: PKC ɛ moved more rapidly than PKC ζ which appeared to translocate more quickly than PKC α. After downregulation of the responsive PKC isoforms with PDBu, the remaining PKC ζ was not translocated by LHRH. (Mol Cell Biochem 165: 65–75, 1996)
Similar content being viewed by others
References
Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC: Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol 6: 1163–1169, 1992
Schrey MP: Gonadotrophin-releasing hormone stimulates the formation of inositol phosphates in rat anterior pituitary tissue. Biochem J 226: 563–569, 1985
Andrews WV, Conn PM: Gonadotropin-releasing hormone stimulates mass changes in phosphoinositides and diacylglycerol accumulation in purified gonadotrope cell cultures. Endocrinology 118: 1148–1158, 1986
Huckle WR, Conn PM: Molecular mechanism of gonadotropin-releasing hormone action. 2. The effector system. Endocr Rev 9: 387–395, 1988
Conn PM, Hawes BE, Janovick JA: Selection of models for the study of GnRH stimulated gonadotropin release prejudices the assignment of role for mediators and modulators of hormone action. Mol Cell Endocrinol 84: C33-C37, 1992
Smith MA, Vale WW: Superfusion of rat anterior pituitary cells attached to cytodex beads: Validation of a technique. Endocrinology 107: 1425–1431, 1980
Thomson FJ, Johnson MS, Mitchell R, Wolbers B, Ison AJ, MacEwan DJ: Differential effects of protein kinase C activators and inhibitors on rat anterior pituitary hormone release. Mol Cell Endocrinol 94: 223–234, 1993
Johnson MS, Thomson FJ, MacEwan DJ, Mitchell R: The involvement of dihydropyridine-sensitive calcium channels in phorbol ester-induced luteinizing hormone and growth hormone release. Mol Cell Endocrinol 95: 31–41, 1993
Johnson MS, Mitchell R, Thomson FJ: The priming effect of luteinizing hormone-releasing hormone (LHRH), but not LHRH-induced gonadotropin release, can be prevented by certain PKC inhibitors. Mol Cell Endocrinol 85: 183–193, 1992
Denef C, Swennen L, Andries M: Separated anterior pituitary cells and their response to hypophysiotropic hormones. Int Rev Cytol 76: 225–244, 1982
Windle JJ, Weiner RI, Mellon PL: Cell lines of the pituitary gonadotrope lineage derived by target oncogenesis in transgenic mice. Mol Endocrinol 4: 597–603, 1990
Johnson MS, MacEwan DJ, Simpson J, Mitchell R: Characterisation of protein kinase C isoforms and enzymic activity from the αT3–1 gonadotroph-derived cell line. FEBS Lett 333: 67–72, 1993
Naor Z, Dan-Cohen H, Hermon J, Limor R: Induction of exocytosis in permeabilized pituitary cells by α and β-type protein kinase C. Proc Natl Acad Sci USA 86: 4501–4504, 1989
Hug H, Sarre TF: Protein kinase C isoenzymes: divergence in signal transduction? Biochem J 291: 329–343, 1993
Wise BC, Glass DB, Chou CH, Raynor RL, Katoh N, Schatzman RC, Turner RC, Kibler RF, Kuo JF: Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II Substrate specificity and inhibition by various agents. J Biol Chem 257: 8489–8495, 1982
Huang K-P, Huang FL, Nakabayashi H, Yoshida Y: Biochemical characterisation of rat brain protein kinase C isozymes. J Biol Chem 263: 14839–14845, 1988
Johnson MS, Simpson J, MacEwan DJ, Ison A, Clegg RA, Connor K, Mitchell R: Phorbol ester and diacylglycerol activation of native protein kinase C species from various tissues. Mol Cell Biochem 146: 127–137, 1995
MacEwan DJ, Johnson MS, Mitchell R, Thomson FJ, Lutz EM, Clegg RA, Connor K: Evidence that protein kinase C α has reduced affinity towards 1,2-dioctanoyl-sn-glycerol: the effects of lipid activators on phorbol ester binding and kinase activity. Eur J Pharmacol Mol Pharmacol Section 246: 9–18, 1993
Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Dukamel D, Charan D, Kirilovsky J: The bisindoylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991
Hidaka H, Hagiwara M: Pharmacology of the isoquinoline sulfonamide Protein Kinase C inhibitors. Trends Pharmacol Sci 8: 162–164, 1987
Limatola C, Schaap D, Moolenaar WH, van Blitterswijk WJ: Phosphatidic acid activation of protein kinase C-ζ overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem J 304: 1001–1008, 1994
Netiv E, Liscovitch M, Naor Z: Delayed activation of phospholipase D by gonadotropin-releasing-hormone in a clonal pituitary gonadotrope cell-line (αT3–1). FEBS Lett 295: 107–109, 1991
Billah MM, Pai J-K, Mullmann TJ, Egan RW, Siegel MI: Regulation of phospholipase D in HL-60 granulocytes. J Biol Chem 264: 9069–9076, 1989
Chen Z-P, Kratzmeier M, Poch A, Xu S, McArdle CA, Levy A, Mukhopadhyay AK, Lightman SL: Effects of extracellular nucleotides in the pituitary: adenosine triphosphate receptor-mediated intracellular responses in gonadotrope-derived αT3- 1 cells. Endocrinology 137: 248–256, 1996
Kiley S, Schaap D, Parker P, Hsieh L-L, Jaken S: Protein kinase C heterogeneity in GH4C1 rat pituitary cells. J Biol Chem 265: 15704–15712, 1990
Chen C-C: Protein kinase C α, δ, ɛ and ζ in C6 glioma cells. FEBS Lett 332: 169–173, 1993
Olivier AR, Parker PJ: Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem 269: 2758–2763, 1994
Bogoyevitch MA, Parker PJ, Sugden PH: Characterization of protein kinase C isotype expression in adult rat heart. Circ Res 72: 757–767, 1993
Standaert ML, Cooper DR, Hernandez H, Arnold TP, Farese RV: Differential down-regulation of insulin-sensitive protein kinase C isoforms by 12–0-tetradecanoyl phorbol-13-acetate in rat adipocytes and BC3H-1 myocytes. Endocrinology 132: 689–692, 1993
Heidenreich KA, Toledo SP, Brunton LL, Watson MJ, Daniel-Issakani S, Strulovici B: Insulin stimulates the activity of a novel protein kinase C, PKC ɛ, in cultured fetal chick neurons. J Biol Chem 265: 15076–15082, 1990
Szallasi Z, Kosa K, Smith CB, Dlugosz AA, Williams EK, Yuspa SH, Blumberg PM: Differential regulation of anti-tumor-promoting 12-deoxyphorbol-13-phenylacetate reveals distinct roles of the classical and novel protein kinase C isozymes in biological responses of primary mouse keratinocytes. Mol Pharmacol 47: 258–265, 1995
Fujihara M, Connolly N, Ito N, Suzuki T: Properties of protein kinase C isoforms (βII, ɛ and ζ) in a macrophage cell line (J774) and their roles in LPS-induced nitric oxide production. J Immunol 152: 1898–1906, 1994
Zhou G, Wooten MW, Coleman ES: Regulation of atypical ζ-protein kinase C in cellular signalling. Exp Cell Res 214: 1–11, 1994
Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y: Protein kinase C ζ subspecies from rat brain: Its structure, expression, and properties. Proc Natl Acad Sci USA 86: 3099–3103, 1989
Huwiler A, Fabbro D, Stabel S, Pfeilschifter J: Immunocharacterization of δ- and ζ-isotrizymes of protein kinase C in rat mesangial cells. FEBS Lett 300: 259–262, 1992
Ways DK, Cook PP, Webster C, Parker PJ: Effect of phorbol esters on protein kinase C-ζ. J Biol Chem 267: 4799–4805, 1992
Gschwendt M, Leibersperger H, Kittstein W, Marks F: Protein kinase Cζ and η in murine epidermis (TPA induces down-regulation of PKCη but not PKCζ. FEBS Lett 307: 151–155, 1992
Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF, Mischak H: The cDNA sequence expression pattern and protein characteristics of mouse protein kinase C-ζ. Gene 122: 305–311, 1992
Crabos M, Fabbro D, Stabel S, Erne P: Effect of tumour-promoting phorbol ester, thrombin and vasopressin on translocation of three distinct protein kinase C isoforms in human platelets and regulation by calcium. Biochem J 288: 891–896, 1992
Borner C, Guadagno SN, Fabbro D, Weinstein IB: Expression of 4 protein kinase C isoforms in rat fibroblasts — distinct subcellular distribution and regulation by calcium and phorbol esters. J Biol Chem 267: 12892–12899, 1992
Wooten MW, Zhou G, Seibenhener ML, Coleman ES: A role for ζ-protein kinase C in nerve growth factor-induced differentiation of PC 12 cells. Cell Growth Differ 5: 395–403, 1994
Allen BG, Andrea JE, Walsh MP: Identification and characterization of protein kinase C ζ-immunoreactive proteins. J Biol Chem 269: 29288–29298, 1994
Nishikawa K, Yamamoto S, Nagumo H, Maruyama K, Kato R: The presence of phorbol ester responsive and non-responsive forms of ζ isozyme of protein kinase C in mouse epidermal cells. Cell Signal 7: 491–504, 1995
Mau SE, Saermark T, Vihardt H: Translocation of protein kinase C isozymes in lactotroph-enriched rat anterior pituitary cell cultures: differential effects of substance P and phorbol ester. J Mol Endocrinol 12: 293–302, 1994
Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, Schächtele C: Selective inhibition of protein kinase C isozymes by the indolocarbazole Go6976. J Biol Chem 268: 9194–9197, 1993
Leach KL, Raben DM: Nuclear location of protein kinase C. Biochem Soc Trans 21: 879–883, 1993
Ha KS, Exton JH: Differential translocation of protein kinase C isozymes by thrombin and platelet-derived growth factor — a possible function for phosphatidylcholine-derived diacylglycerol. J Biol Chem 268: 10534–10539, 1993
Davidson JS, Wakefield IK, Millar RP: Absence of rapid desensitization of the mouse gonadotropin-releasing hormone receptor. Biochem J 300: 299–302, 1994
Khalil RA, Lajoie C, Resnick MS, Morgan KG: Ca2+-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol 263: C714–719, 1992
Berti L, Mosthaf L, Kroder G, Kellerer M, Tippmer S, Mushak J, Seffer E, Seedorf K, Häring H: Glucose-induced translocation of protein kinase C isoforms in Rat-1 fibroblasts is paralleled by inhibition of the insulin receptor tyrosine kinase. J Biol Chem 269: 3381–3386, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johnson, M.S., Simpson, J. & Mitchell, R. Effect of phorbol 12,13-dibutyrate on ligand binding, enzyme activity and translocation of protein kinase C isoforms in the αT3-1 gonadotrope-derived cell line. Mol Cell Biochem 165, 65–75 (1996). https://doi.org/10.1007/BF00229746
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229746