Abstract
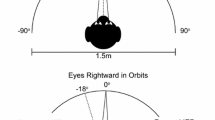
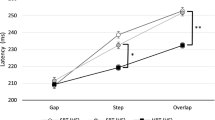
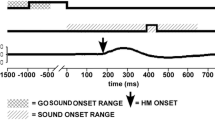
We studied the characteristics of combined eye-head gaze shifts in human subjects to determine whether they used similar strategies when looking at visual (V), auditory (A), and combined (V+A) targets located at several target eccentricities along the horizontal meridian. Subjects displayed considerable variability in the combinations of eye and head movement used to orient to the targets, ranging from those who always aligned their head close to the target, to those who relied predominantly on eye movements and only moved their head when the target was located beyond the limits of ocular motility. For a given subject, there was almost no variability in the amount of eye and head movement in the three target conditions (V, A, V+A). The time to initiate a gaze shift was influenced by stimulus modality and eccentricity. Auditory targets produced the longest latencies when located centrally (less than 20° eccentricity), whereas visual targets evoked the longest latencies when located peripherally (greater than 40° eccentricity). Combined targets (V+A) elicited the shortest latency reaction times at all eccentricities. The peak velocity of gaze shifts was also affected by target modality. At eccentricities between 10 and 30°, peak gaze velocity was greater for movements to visual targets than for movements to auditory targets. Movements to the combined target were of comparable speed with movements to visual targets. Despite the modality-specific differences in reaction latency and peak gaze velocity, the consistency of combinations of eye and head movement within subjects suggests that visual and auditory signals are remapped into a common reference frame for controlling orienting gaze shifts. A likely candidate is the deeper layers of the superior colliculus, because visual and auditory signals converge directly onto the neurons projecting to the eye and head premotor centers.
Similar content being viewed by others
References
Barmack NH, Bell CC, Rence BG (1971) Tension and rate of tension development during isometric responses of extraocular muscle. J Neurophysiol 34:1072–1079
Barnes G (1979) Vestibulo-ocular function during coordinated head and eye movements to acquire visual targets. J Physiol (Lond) 287:127–147
Botterman BR, Iwamoto GA, Gonyea WJ (1986) Gradation of isometric tension by different activation rates in motor units of cat flexor carpi radialis muscle. J Neurophysiol 56:494–506
Cowie RJ, Robinson DL (1994) Subcortical contributions to head movements in macaques. I. contrasting effects of electrical stimulations of a medial pontomedullary region and the superior colliculus. J Neurophysiol 72(6):2648–2664
Dorris MC, Ballantyne PA, Munoz DP (1993) Evidence for a common drive signal in combined eye-head movements in humans. Neurosci Abstr 19:789
Dorris MC, Goldring JE, Corneil BD, Munoz DP (1994) Strategies and characteristics of human head-free gaze shifts (abstract). Can J Physiol Pharmacol 72(2):Avi
Engelken EJ, Stevens KW (1989) Saccadic eye movements in response to visual, auditory, and bisensory stimuli. Aviat Space Environ Med 60:762–768
Frens MA, Van Opstal AJ (1995) A quantitative study of auditory-evoked saccadic eye movements in two dimensions. Exp Brain Res 107:103–117
Frens MA, Van Opstal AJ, Van der Willigen RF (1995) Spatial and temporal factors determine audio-visual interactions in human saccadic eye movements. Percept Psychophys 57:802–816
Fuller JH (1992) Head movement propensity. Exp Brain Res 92:152–164
Grantyn A, Berthoz A (1985) Burst activity of identified tecto-reticulo-spinal neurons in the alert cat. Exp Brain Res 57:417–421
Gretsy M (1974) Coordination of head and eye movements to fixate continuous and intermittent targets. Vision Res 14:395–403
Guitton D, Munoz DP (1991) Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. I. Identification, localization, and effects of behavior on sensory responses. J Neurophysiol 66:1605–1623
Guitton D, Volle M (1987) Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol 58:427–459
Hays AV, Richmond BJ, Optician LM (1982) A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc 2:1–10
Hirsch JA, Chan JCK, Yin TCT (1985) Responses of neurons in the cat's superior colliculus to acoustic stimuli. I. Monaural and binaural response properties. J Neurophysiol 53:726–745
Hughes HC, Reuter-Lorenz PA, Nozawa G, Fendrich R (1994) Visual-auditory interactions in sensorimotor processing: saccades versus manual responses. J Exp Psychol Gen 20:131–153
Jay MF, Sparks DL (1987) Sensorimotor integration in the primate superior colliculus. II. Coordinates of auditory signals. J Neurophysiol 57:35–55
Kalesnykas RP, Hallett PE (1994) Retinal eccentricity and the latency of eye saccades. Vision Res 34:517–531
Land MF (1992) Preictable eye-head coordination during driving. Nature 359:318–320
Lueck CJ, Crawford TJ, Savage CJ, Kennard C (1990) Auditoryvisual interaction in the generation of saccades in man. Exp Brain Res 82:149–157
Mays LE, Sparks DL (1980) Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol 43:207–232
Merideth MA, Stein BE (1986) Visual, auditory and somatosensory convergence on cells in superior colliculus results in multi-sensory integration. J Neurophysiol 56:640–662
Merideth MA, Nemitz JW, Stein BE (1987) Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci 7:3215–3229
Middlebrooks JC, Knudsen EI (1984) A neural code for auditory space in the cat's superior colliculus. J Neurosci 4:2621–2634
Munoz DP, Guitton D, Pelisson D (1991) Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharge. J Neurophysiol 66:1642–1666
Olivier E, Grantyn A, Chat M, Berthoz A (1993) The control of slow orienting eye movements by tectoreticulospinal neurons in the catbehavior, discharge patterns and underlying connections. Exp Brain Res 93:435–449
Paré M, Crommelink M, Guitton D (1994) Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res 101:123–139
Peck CK (1987) Visual-auditory interactions in cat superior colliculus: their role in the control of gaze. Brain Res 420:162–166
Peck CK, Schlag-Rey M, Schlag J (1980) Visuo-oculomotor properties of cells in the superior colliculus of the alert cat. J Neurophyiol 70:576–589
Ron S, Berthoz A (1991) Eye and head coupled and dissociated movements during orientation to a double step visual target displacement. Exp Brain Res 85:196–207
Ron S, Berthoz A, Gur S (1993) Saccade-vestibulo-ocular reflex co-operation and eye-head uncoupling during orientation to flashed target. J Physiol (Lond) 464:595–611
Roucoux AD, Guitton D, Crommelinck M (1980) Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Exp Brain Res 39:75–85
Segraves MA, Goldberg ME (1992) Properties of eye and head movements evoked by electrical stimulation of the monkey superior colliculus. In: Berthoz A, Vidai PP, Werner G (eds) The head-neck sensory motor system. Oxford University Press, New York, pp 292–295
Stein BE, Merideth MA, Huneycutt WS, McDade L (1989) Behavioral indices of multisensory integration: orientation to visual cues is affected by auditory stimuli. J Cogn Neurosci 1:12–24
Syka J, Popelar J, Bozkov V (1979) Responses of neurons in the superior colliculus of the cat to stationary and moving visual stimuli. Vision Res 19:213–219
Volle M, Guitton D (1993) Human gaze shifts in which head and eyes are not initially aligned. Exp Brain Res 94:463–470
Wise LZ, Irvine DRF (1983) Auditory response properties of neurons in deep layers of cat superior colliculus. J Neurophysiol 49:674–685
Zahn JR, Abel LA, Dell'Osso LF (1978) Audio-ocular response characteristics. Sensory Processes 2:32–37
Zambarbieri D, Schmid R, Magenes G, Prablanc C (1982) Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res 47:417–427
Zangemeister WH, Stark L (1982a) Gaze latency: variable interactions of head and eye latency. Exp Neurol 75:389–406
Zangemeister WH, Stark L (1982b) Types of gaze movements: Variable interactions of eye and head movements. Exp Neurol 77:563–577
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goldring, J.E., Dorris, M.C., Corneil, B.D. et al. Combined eye-head gaze shifts to visual and auditory targets in humans. Exp Brain Res 111, 68–78 (1996). https://doi.org/10.1007/BF00229557
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229557