Summary
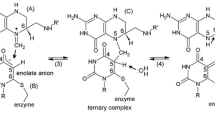
The complexes that thymidylate synthetase (TSase) forms with various potent inhibitors have been intensively studied and thoroughly reviewed. Of particular significance is the covalent ternary complex of TSase-FdUMP-5,10-CH2H4PteGlu. HUMP is the active metabolite of the widely used anti-cancer drug 5-fluorouracil. This complex is thought to be analogous to a steady-state intermediate of the normal enzyme reaction with the substrate dUMP. In this review, we examine the properties of TSase-dUMP complexes in order to determine if there is an experimental basis for drawing a close analogy between dUMP and HUMP in their interaction with TSase, and also to evaluate data indicating a potential chemotherapeutic value for TSase-dUMP complexes formed in the presence of folate analogs.
Similar content being viewed by others
Abbreviations
- TSase:
-
thymidylate synthetase
- 5,10-CH2H4Pte-Glu:
-
5,10-methylenetetrahydrofolic acid
- dUMP:
-
2′-deoxyuridine-5′phosphate
- FdUMP:
-
5-fluoro-2′deoxyuridine-5′-phosphate
- dTMP:
-
thymidylic acid
- H2PteGlu:
-
dihydrofolic acid
- BrdUMP:
-
5-bromo-2′-deoxyuridylic acid
- 4-N-hydroxy dCMP:
-
4-N-hydroxy-2′-deoxycytidylic acid
- MTX:
-
methotrexate
- PteGlu3 :
-
pteroic acid triglutamate
- 5-FUra:
-
5-fluorouracil
- DTNB:
-
2,2′-dithiobisnitrobenzoic acid
- NEM:
-
N-ethylmaleimide
References
Humphreys, G. K. & Greenberg, D. M., 1958. Arch. Biochem. Biophys. 78: 275–287.
Friedkin, M., 1959. Fed. Proc. Fed. Am. Soc. Exp. Biol. 18: 230.
Blakley, R. L., 1960. The Biochemistry of Folic Acid and Related Pteridines, American Elsevier, New York, p. 237.
Danenberg, P. V., 1977. Biochim. Biophys. Acta 473: 73–92.
Cohen, S. S., Flaks, J. G., Barner, H. D., Loeb, M. R. & Lichtenstein, J., 1958. Proc. Natl. Acad. Sci. U.S.A. 44: 1004–1012.
Langenbach, R. J., Danenberg, P. V. & Heidelberger, C., 1972. Biochem. Biophys. Res. Commun. 48: 1565–1571.
Cleland, W. W., 1967. Adv. Enzymol. 29: 1–65.
Blakley, R. L., 1963. J. Biol. Chem. 238: 2113–2118.
Reyes, P. & Heidelberger, C., 1965. Mol. Pharmacol. 1: 14–30.
Cha, S., 1975. Biochem. Pharmacol. 24: 2177–2185.
Lorenson, M. Y., Maley, C. F. & Maley, F., 1967. J. Biol. Chem. 242: 3332–3344.
Santi, D. V., Pogolotti, A. L., James, T. L., Wataya, Y., Ivanetich, K. M. & Lam, S. S. M., 1976. In: Biochemistry Involving Carbon-Fluorine Bonds (Filler, R., ed.). ACS Symposium Series No. 28, American Chemical Society Washington, D.C., pp. 57–75.
Danenberg, P. V. & Danenberg, K. D., 1978. Biochemistry 17:4018–4024.
Slavik, K. & Zakrzewski, S. F., 1967. Mol. Pharmacol. 3: 370–376.
Fromm, H. J., 1967. Biochim. Biophys. Acta 139: 221–227.
Danenberg, K. D. & Cleland, W. W., 1975. Biochemistry 15: 28–39.
Lockshin, A. & Danenberg, P. V., 1981. Biochem. Pharmacol. 30: 247–257.
Daron, H. H. & Aull, J. L., 1978. J. Biol. Chem. 253: 940–945.
Galivan, J. H., Maley, F. & Baugh, C. M., 1975. Biochem. Biophys. Res. Commun. 71: 527–534.
Galivan, J. H., Maley, G. F. & Maley, F., 1976. Biochemistry 15:356–362.
Danenberg, P. V., Langenbach, R. J. & Heidelbergen, C., 1974. Biochemistry 13: 926–933.
Santi, D. V., McHenry, C. S. & Sommer, H., 1974. Biochemistry 13: 471–480.
Lockshin, A. & Danenberg, P. V., 1979. J. Biol. Chem. 254: 12285–12288.
Fernandes, D. J. & Bertino, J. R., 1980. Proc. Natl. Acad. Sci. U.S.A. 77: 5663–5667.
Slavik, K., Rode, W. & Slavikova, V., 1976. Biochemistry 15:4222–4227.
Lockshin, A., Moran, R. G. & Danenberg, P. V., 1979. Proc. Natl. Acad. Sci. U.S.A. 76: 750–754.
Dolnick, B. J. & Cheng, Y.-C., 1977. J. Biol. Chem. 252: 7697–7703.
Plante, L. T., Gaumont, Y. & Kislivk, R. L., 1978. Prep. Biochem. 8: 91–98.
Priest, D. G., Doig, M. T. & Hynes, J. B., 1981. Experientia 37:119–120.
Maley, G. F., Bellisario, R. L., Guarino, D. V. & Maley, F., 1979. J. Biol. Chem. 254: 1301–1304.
Galivan, J., Noonan, J. & Maley, F., 1977. Arch. Biochem. Biophys. 184: 336–345.
Plese, P. C. & Dunlap, R. B., 1977. J. Biol. Chem. 252: 6139–6144.
Danenberg, K. D. & Danenberg, P. V., 1979. J. Biol. Chem. 254:4345–4348.
Dunlap, R. B., Harding, N. G. L. & Huennekens, F. M., 1971. Biochemistry 10: 88–97.
Leary, R. P., Beaudette, N. & Kisliuk, R. L., 1975. J. Biol. Chem. 250: 4864–4868.
Beaudette, N. V., Langerman, N., Kislivk, R. L. & Gaumant, Y., 1977. Arch. Biochem. Biophys. 179: 272–278.
Hummel, J. P. & Dreyer, W. J., 1962. Biochim. Biophys. Acta 63: 530–532.
Scatchard, G., 1949. Ann. N.Y. Acad. Sci. 51: 660–672.
Bellisario, R. L., Maley, G. F., Galivan, J. H. & Maley, F., 1976. Proc. Natl. Acad. Sci. U.S.A. 73: 1848–1852.
Bellisario, R. L., Maley, G. F., Guarino, D. V. & Maley, F. M., 1979. J. Biol. Chem. 254: 1296–1300.
Lazdunski, M., Petitclerc, C., Chappelet, D. & Lazdunski, C., 1971. Eur. J. Biochem. 20: 124–139.
Hayatsu, H., Wataya, Y. & Kai, K., 1970. J. Amer. Chem. Soc. 92: 724–726.
James, T. L., Pogolotti, Jr., A. L., Ivanetich, K. M., Wataya, Y., Lam, S. S. M. & Santi, D. V., 1976. Biochem. Biophys. Res. Commun. 72: 404–409.
Byrd, R. A., Dawson, W. H., Ellis, P. D. & Dunlap, R. B., 1977. J. Amer. Chem. Soc. 99: 6139–6141.
Byrd, R. A., Dawson, W. H., Ellis, P. D. & Dunlap, R. B., 1978. J. Amer. Chem. Soc. 100: 7478–7486.
Lewis, C. A., Ellis, P. D. & Dunlap, R. B., 1981. Biochemistry 20: 2275–2285.
Pogolotti, Jr., A. L., Weill, C. & Santi, D. V., 1979. Biochemistry 18: 2794–2798.
Santi, D. V. & Brewer, C. F., 1968. J. Amer. Chem. Soc. 90: 6236–6238.
Cipollo, K. L. & Dunlap, R. B., 1978. Biochem. Biophys. Res. Commun. 81: 1139–1144.
Beckage, M. J., Blumenstein, M. & Kisliuk, R. L., 1979. Biochim. Biophys. Acta 571: 157–161.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Danenberg, P.V., Lockshin, A. Thymidylate synthetase — substrate complex formation. Mol Cell Biochem 43, 49–57 (1982). https://doi.org/10.1007/BF00229539
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00229539