Summary
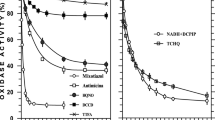
Redox inactivation of glutathione reductase involves metal cations, since chelators protected against NADPH-inactivation, 3 µM EDTA or 10 µM DETAPAC yielding full protection. Ag+, Zn2+ and Cd2+ potentiated the redox inactivation promoted by NADPH alone, while Cr3+, Fe2+, Fe3+, Cu+, and Cu2+ protected the enzyme. The Zn2+ and Cd2+ effect was time-dependent, unlike conventional inhibition. Glutathione reductase interconversion did not require dioxygen, excluding participation of active oxygen species produced by NADPH and metal cations. One Zn2+ ion was required per enzyme subunit to yield full NADPH-inactivation, the enzyme being reactivated by EDTA. Redox inactivation of glutathione reductase could arise from the blocking of the dithiol formed at the active site of the reduced enzyme by metal cations, like Zn2+ or Cd2+.
The glutathione reductase activity of yeast cell-free extracts was rapidly inactivated by low NADPH or moderate NADH concentrations; NADP+ also promoted rapid inactivation in fresh extracts, probably after reduction to NADPH. Full inactivation was obtained in cell-free extracts incubated with glucose-6-phosphate or 6-phosphogluconate; the inactivating efficiency of several oxidizable substrates was directly proportional to the specific activities of the corresponding dehydrogenases, confirming that redox inactivation derives from NADPH formed in vitro.
Similar content being viewed by others
Abbreviations
- DETAPAC:
-
diethylenetriaminepentaacetic acid
- 2′,5′-ADP-Sepharose-N6-(6-aminohexyl) adenosine:
-
2′,5′-bisphosphateSepharose
References
Williams CH Jr: Flavin-containing dehydrogenases and oxidases. In: PD Boyer (ed) The Enzymes vol XIII, Academic Press, New York. 1976, pp 89–173
Krauth-Siegel RL, Blatterspiel R, Saleh M, Schiltz E, Schirmer RH, Untücht-Grau R: Glutathione reductase from human erythrocytes. The sequences of the NADPH domain and of the interface domain. Eur J Biochem 121: 259–267, 1982
Thieme R, Pai EF, Schirmer RH, Schulz GE: Three-dimensional structure of glutathione reductase at 2 Å resolution. J Mol Biol 152: 763–782, 1981
Karplus PA, Schulz GE: Refined structure of glutathione reductase at 1.54 Å resolution. J Mol Biol 195: 701–729, 1987
Pai EF, Karplus PA, Schulz GE: Crystallographic analysis of the binding of NADPH, NADPH fragments, and NADPH analogues to glutathione reductase. Biochemistry 27: 4465–4474, 1988
Karplus PA, Pai EF, Schultz GE: A crystallographic study of the glutathione binding site of glutathione reductase at 0.3 nm resolution. Eur J Biochem 178: 693–703, 1989
Karplus PA, Schulz GE: Substrate binding and catalysis by glutathione reductase as derived from refined enzyme: substrate crystal structures at 2 Å resolution. J Mol Biol 210: 163–180, 1989
Pai EF, Schulz GE: The catalytic mechanism of glutathione reductase as derived from X-ray diffraction analyses of reaction intermediates. J Biol Chem 258: 1752–1757, 1983
Worthington DJ, Rosemeyer MA: Glutathione reductase from human erythrocytes. Catalytic properties and aggregation. Eur J Biochem 67: 231–238, 1976
López-Barea J, Lee, C-Y: Mouse-liver glutathione reductase. Purification, kinetics and regulation. Eur J Biochem 98: 487–499, 1979
Pinto MC, Mata AM, López-Barea J: Reversible inactivation of S. cerevisiae glutathione reductase under reducing conditions. Arch Bioch Biophys 228: 1–12, 1984
Pinto MC, Mata AM, López-Barea J: The redox interconversion mechanism of Saccharomyces cerevisiae glutathione reductase. Eur J Biochem 151: 275–281, 1985
Mata AM, Pinto MC, López-Barea J: Redox interconversion of glutathione reductase from Escherichia coli. A study with pure enzyme and cell-free extracts. Mol Cell Biochem 67: 65–76, 1985
Mata AM, Pinto MC, López-Barea J: Redox interconversion of Escherichia coli glutathione reductase. A study with permeabilized and intact cells. Mol Cell Biochem 68: 121–130, 1985
López-Barea J, Bárcena JA: Redox control of glutathione and thioredoxin reductases. In: FL Crane, DJ Morre, HA Low (eds) Plasma membrane oxidoreductases in control of animal and plant growth. Plenum Publishing Corp, New York, 1988, pp 349–358
López-Barea J, Barcena JA, Bocanegra JA, Florindo J, García-Alfonso C, López-Ruiz A, Martínez-Galisteo E, Peinado J: Structure, mechanism, functions, and regulatory properties of glutathione reductase. In: J Viña (ed) in CRC Handbook of Glutathione: Metabolism and Physiological Functions. CRC Press, Boca Raton, in press
Greer S, Perham RN: Glutathione reductase from Escherichia coli: cloning and sequence analysis of the gene and relationship to other flavoprotein disulphide oxidoreductases. Biochemistry 25: 2736–2742, 1986
Berry A, Scrutton NS, Perham RN: Switching kinetic mechanism and putative proton donor by directed mutagenesis of glutathione reductase. Biochemistry 28: 1264–1269, 1989
Deonarain MP, Berry A, Scrutton NS, Perham RN: Alternative proton donors-acceptors in the catalytic mechanism of the glutathione reductase of Escherichia coli. The role of histidine-439 and tyrosine-99. Biochemistry 28: 9602–9607, 1989
Scrutton NS, Berry A, Perham RN: Engineering of an intersubunit disulphide bridge in glutathione reductase from Escherichia coli. FEBS Lett 241: 46–50, 1988
Arscott LD, Drake DM, Williams CH Jr: Inactivation of two-electron reduced Escherichia coli glutathione reductase by NADPH and NADH. In: DE Edmonson, DB McCormick (eds) Flavins and Flavoproteins 1987. Walter de Gruyter, Berlin, 1987, pp 75–78
Arscott LD, Drake DM, Williams CH Jr: Inactivationreactivation of two-electron reduced Escherichia coli glutathione reductase involving a dimer-monomer equilibrium. Biochemistry 28: 3591–3598, 1989
Serrano A, Rivas J, Losada M: Purification and properties of glutathione reductase from the cyanobacterium Anabaena sp. Strain 7119. J Bacteriol 158: 317–324, 1984
Connell JP, Mullet JE: Pea chloroplast glutathione reductase: purification and characterization. Plant Physiol 82: 351–356, 1986
Mize CE, Langdom RG: Hepatic glutathione reductase. I. Purification and general kinetic properties. J Biol Chem 237: 1589–1595, 1962
Chung YC, Hurlbert RE: Purification and properties of glutathione reductase of Chromatium vinosum. J Bacteriol 123: 203–211, 1975
Halliwell B, Foyer CH: Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 139: 9–17, 1978
Rafter GW: Copper inhibition of glutathione reductase and its reversal with gold thiolates, thiol, and disulfide compounds. Biochem Med 27: 381–391, 1982
Shigeoka S, Onishi T, Nakano Y, Kitaoka S: Characterization and physiological function of glutathione reductase in Euglena gracilis z. Biochem J 242: 511–515, 1987
Davis BJ: Disc electrophoresis II. Method and application to human serum proteins. Ann NY Acad Sci 121: 404–427, 1964
Lohor GW, Walter HD: Glucose-6-phosphate dehydrogenase. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis 2nd ed. Academic Press, New York, 1974, pp 636–643
King J: 6-Phosphogluconate dehydrogenase. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis 2nd ed. Academic Press, New York, 1974, pp 632–635
Bergmeyer HU, Bernt E: Isocitrate dehydrogenase. UV-assay. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis 2nd ed. Academic Press, New York, 1974, pp 625–627
Kornberg A, Pricer WE Jr: Di- and triphosphopyridine nucleotide isocitric dehydrogenase in yeast. J Biol Chem 189: 123–136, 1951
Bergmeyer HU, Bernt E: Malate dehydrogenase. UV-assay. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis 2nd ed. Academic Press, New York, 1974, pp 613–617
Bergmeyer HU, Bernt E: Lactate dehydrogenase. UV-assay with pyruvate and NADH. In: HU Bergmeyer (ed) Methods of Enzymatic Analysis 2nd ed. Academic Press, New York, 1974, pp 574–579
Lowry OH, Rosebrough MJ, Farr AL, Randall RJ: Protein measurements with the Folin-phenol reagent. J Biol Chem 193: 265–275, 1951
Buettner GR, Doherty TP, Patterson LK: The kinetics of the reaction of superoxide radical with Fe(III) complexes of EDTA, DETAPAC and HEDTA. FEBS Lett 158: 143–146, 1983
Jocelyn PC: Biochemistry of the SH Group. Academic Press, London, 1972, pp 94–100
Bochner BR, Lee PC, Wilson SW, Cutler CW, Ames BN: AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell 37: 225–232, 1984
Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN: Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci USA 83: 8059–8063, 1986
Zamecnik P: Diadenosine 5′,5′"-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem 134: 1–10, 1983
Malmström BG: Enzymology of oxygen. Ann Rev Biochem 51: 21–59, 1982
Dunford HB: Free radicals in iron-containing systems. Free Rad Biol 3: 405–421, 1987
Sies H: Biochemistry of oxidative stress. Angew Chem Int Ed Engl 25: 1058–1071, 1986
Klug A, Rhodes D: ‘Zinc fingers’: a novel protein motif for nucleic acid recognition. Trends Biochem Sci 12: 464–469, 1987
Llobell A, López-Ruiz A, Peinado J, López-Barea J: Glutathione reductase directly mediates the stimulation of yeast glucose-6-phosphate dehydrogenase by GSSG. Biochem J 249: 293–296, 1988
Kosower NS, Kosower EM: The glutathione status of the cell. Int Rev Cytol 54: 109–160, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peinado, J., Florindo, J., García-Alfonso, C. et al. Metals are directly involved in the redox interconversion of Saccharomyces cerevisiae glutathione reductase. Mol Cell Biochem 101, 175–187 (1991). https://doi.org/10.1007/BF00229534
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229534