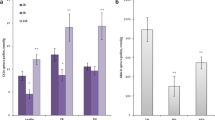
Abstract
The dry type stigma of Brassica is covered with a continuous layer of cuticle. Cutinase and non-specific esterases may be involved in breakdown of this cuticle barrier during pollen-stigma interaction, but only a little is known about their nature and characteristics. We report here the presence of two distinct esterases from stigma and pollen of Brassica. A 33 kD esterase assayed using MU-butyrate substrate shows high activity in stigma papillae. A similar esterase from Tropaeolum pollen has been shown to possess active cutinase activity. The esterase activity in anther tissue is due to a 24 kD enzyme with substrate specificity toward acetate esters. Both enzymes require sulfhydryl groups for their catalytic activity. Immunogold labelling of antibodies raised against these esterases localised the proteins at the subcellular level. Antibodies for MU-butyrate hydrolase gave a positive signal in the cell walls of mature stigma papillae and in the tapetum and microspores during early stages of anther development. In the mature anther, a positive signal in the cytoplasm of pollen grains with some detectable localisation in the exine layer of the pollen wall was obtained. Similar results were obtained with acetate hydrolase antibodies. These esterases are thus spatially and temporally regulated in stigma and anther tissues.
Similar content being viewed by others
Abbreviations
- MU :
-
methyl umbelliferyl
- pAbC :
-
anti-butyrate hydrolase polyclonal antibodies
- pAbE :
-
anti-acetate hydrolase polyclonal antibodies
References
Ansorge W (1983) Fast visualisation of protein bands by impregnation in potassium permanganate and silver nitrate. In: Stathakos D (ed) Electropheresis '82. Advanced methods, biochemical and clinical applications. Walter de Fruyter, Berlin, pp 235–242
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254
Brewbaker JL (1971) Pollen enzymes and isoenzymes. In: Heslop-Harrison J (ed) Pollen: development and physiology. Butterworths, London, pp 156–170
De Block M, Debrouwer D (1992) In-situ enzyme histochemistry on plastic-embedded plant material. The development of an artefact-free β-glucuronidase assay. Plant J 2(2): 261–266
Dhaliwal AS, Malik CP (1982) Release of esterases from incompatible Brassica campestris L. pollen in vitro & in vivo. Indian J Exp Biol 20:95–96
Evans DE (1990) Potential for pollen selection in oilseed rape. PhD thesis, University of Melbourne, Australia
Ferrari TE, Best V, More TA, Comstock P, Muhammad A, Wallace DH (1985) Intercellular adhesions in the pollen-stigma system: pollen capture, grain binding and tube attachment. Am J Bot 72:1466–1474
Heinen W, Linskens HF (1961) Enzymic breakdown of stigmatic cuticle of flowers. Nature 191:1416
Heslop-Harrison J (1975) Incompatibility and the pollen-stigma interaction. Annu Rev Plant Physiol 26:403–425
Heslop-Harrison J, Heslop-Harrison Y, Barber J (1975a) The stigma surface in incompatibility responses. Proc R Soc London Ser B 188:287–297
Heslop-Harrison J, Knox RB, Heslop-Harrison Y, Mattsson O (1975b) Pollen-wall proteins: emission and role incompatibility responses. In: Duckett JG, Racey PA (eds) The biology of the male gamete. Biol J Linn Soc [Suppl 1] 7: 189–202
Hiscock SJ, Dewey FM, Coleman JOD, Dickinson HG (1994) Identification and localization of an active cutinase in the pollen of Brassica napus L. Planta 193:377–384
Kandasamy TMK, Nasrallah ME, Nasrallah JB (1993) Genetic ablation of floral cells in Arabidopsis. Plant Cell 5:253–261
Knox RB (1984) The pollen grain. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York, pp 197–271
Knox RB, Heslop-Harrison J (1971) Pollen-wall proteins: localisation of antigenic and allergenic proteins in the pollen grain walls of ambrosia spp. (rag-weeds) Cytobios 4:49–54
Knox RB, Heslop-Harrison J, Heslop-Harrison Y (1975) Pollen-wall proteins. In: Duckett JG, Racey PA (eds) The biology of the male gamete. Academic Press, London, pp 177–187
Knox RB, Heslop-Harrison J, Reed C (1970) Localisation of antigens associated with the pollen grain wall by immunofluorescence. Nature 225:1066–1068
Knox RB, Clarke A, Harrison S, Smith P, Marchalonis JJ (1976) Cell recognition in plants: Determinants of the stigma surface and their pollen interactions. Proc Natl Acad Sci USA 73:2788–2792
Kolattukudy PE (1985) Enzymatic penetration of the plant cuticle by fungal pathogens. Ann Rev Phytopathol 23:223–250
Kolattukudy PE, Purdy RE, Maiti IB (1981) Cutinases from fungi and pollen. Methods Enzymol 71:652–664
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lavithis M (1994) Developmental biology of Brassica and Arabidopsis pollen. MSc thesis, University of Melbourne, Australia
Maiti IB, Kolattukudy PE, Shaykh M (1979) Purification and characterization of a novel cutinase from nasturtium (Tropaeolum majus) pollen. Arch Biochem Biophys 196:412–423
Nakanishi T, Esashi Y, Hinata K (1969) Control of self-incompatibility by CO2 gas in Brassica. Plant Cell Physiol 10:925–927
O'Neil P, Singh MB, Neales TF, Knox RB, Williams EG (1984) Carbon dioxide blocks the stigma callose response following incompatible pollinations in Brassica. Pl Cell Environ 7:285–288
O'Neil P, Singh MB, Knox RB (1988) Cell biology of the stigma of Brassica campestris in relation to CO2 effects on self-pollination. J Cell Sci 89:541–549
Pedersen S, Simonsen V, Loeschcke V (1987) Overlap of gametophytic and sporophytic gene expression in barley. Theor Appl Genet 75:200–206
Purdy RE, Kolattukudy PE (1973) Depolymerization of a hydroxyfatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi: isolation and some properties of the enzyme. Arch Biochem Biophys 159:61–69
Purdy RE, Kolattukudy PE (1975) Hydrolysis of plant cuticle by plant pathogens. Purification, amino acid composition, and molecular weight of two isozymes of cutinase and a non-specific esterase from Furasium solani f. pisi. Biochemistry 14 (13): 2824–2840
Sari Gorla M, Frova C, Binelli G, Ottaviano E (1986) The extent of gametophytic-sporophytic gene expression in maize. Theor Appl Genet 72:42–47
Shaykh M, Kolattukudy PE, Davis R (1977a) Production of a novel extracellular cutinase by the pollen and the chemical composition and ultrastructure of the stigma cuticle of nasturtium (Tropaeolum majus). Plant Physiol 60:907–915
Shaykh M, Soliday C, Kolattukudy PE (1977b) Proof of the production of cutinase by Fusarium solani f. pisi during penetration into its host, Pisum sativum. Plant Physiol 60:170–172
Shivanna KR, Heslop-Harrison J, Heslop-Harrison Y (1978) The pollen-stigma interaction: bud pollination in the Cruciferae. Acta Bot Neerl 27:107–119
Singh MB, Taylor PE, Knox RB (1993) Special preparation methods for immunocytochemistry of plant cells. In: Beesley JE (ed) Immunocytochemistry: a practical approach. Oxford IRL Press, Oxford, pp 77–102
Soliday Cl, Kolattukudy PE (1976) Isolation and characterization of a cutinase from Fusarium roseum culmorum and its immunological comparison with cutinases from F. solani pisi. Arch Biochem Biophys 176:334–343
Staff IA, Taylor PE, Smith P, Singh MB, Knox RB (1990) Cellular localization of water soluble, allergenic proteins in rye-grass (Lolium perenne) pollen using monoclonal and specific IgE antibodies with immunogold probes. Histochem J 22:276–290
Tanabe K, Nishimura S, Kohmoto K (1988) Cutinase production by Alternaria alternata Japanese pear pathotype and its role in pathogenicity. Ann Phyopathol Soc Jpn 54:483–492
Tanksley SD, Orton TJ (eds) (1983) Isozymes in plant genetics and breeding, part B. Elsevier, New York
Tanksley SD, Zamir D, Rick CM (1981) Evidence for extensive overlap of sporophytic and gametophytic gene expression in Lycopersicon esculentum. Science 213:453–455
Tashian RE (1969) The esterases and carbonic anhydrases of human erythrocytes. In: Yunis JJ (ed) Biochemical methods in red cell genetics. Academic Press, New York, pp 307–336
Taylor PE, Staff IA, Singh MB, Knox RB (1994) Localization of the two major allergens in rye-grass pollen using specific monoclonal antibodies and quantitative analysis of immunogold labelling. Histochem J 26:392–401
Theerakulpisut P (1990) Gene expression during pollen development in Brassica campestris L. and Lolium perenne L. PhD thesis, School of Botany, University of Melbourne
Theerakulpisut P, Xu H, Singh MB, Pettitt JM, Knox RB (1991) Isolation and developmental expression of Bcp1, an antherspecific cDNA clone in Brassica campestris. Plant Cell 3:1073–1084
Vithanage HIMV, Knox RB (1976) Pollen-wall proteins: quantitative cytochemistry of the origins of intine and exine enzymes in Brassica oleracea. J Cell Sci 21:423–435
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lavithis, M., Bhalla, P.L. Esterases in pollen and stigma of Brassica . Sexual Plant Reprod 8, 289–298 (1995). https://doi.org/10.1007/BF00229386
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229386