Abstract
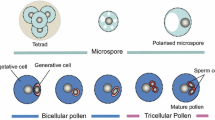
The localization of transcripts of the pollenspecific gene NTP303 has been determined by means of in situ hybridization in combination with confocal laser scanning microscopy. NTP303 transcripts were first detectable at the mid-bi-nucleate stage of pollen development and persisted even after the pollen tube had reached the ovary. The majority of the NTP303 transcripts were localized in the vegetative cell, and were predominantly present in the apex of the pollen tube. To study the mechanism of regulation, the genomic clone NTPg303 was isolated and characterized. NTPg303 belongs to a gene family of at least five members. No introns are present in its coding region. Regions matching the pollen-specific PB-element TGTGGTT (Twell et al. 1991) have been found.
Similar content being viewed by others
References
Albani D, Sardana R, Robert LS, Altosaar I, Arnison PG, Fabijanski SF (1992) A Brassica napus gene family which shows sequence similarity to ascorbate oxidase is expressed in developing pollen molecular characterization and analysis of promoter activity in transgenic tobacco plants. Plant J 2:331–342
Bassell GJ (1993) High resolution distribution of mRNA within the cytoskeleton. J Cell Biochem 52:127–133
Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4:879–887
Bernatzky R, Tanksley SD (1986) Genetics of actin related sequences in tomato. Theor Appl Genet 72:314–321
Blackmore S, Knox RB (eds) (1990) Microspores evolution and ontogeny. Academic press, London
Buuren M van, Neuhaus J-M, Shinshi H, Ryals J, Meins Jr F (1992) The structure and regulation of homeologous tobacco endochitinase genes of Nicotiana sylvestris and N. tomentosiformis origin. Mol Gen Genet 232:460–469
Carpenter JL, Ploense SE, Snustad DP, Silflow CD (1992) Preferential expression of an alpha-tubulin gene of Arabidopsis in pollen. Plant Cell 4:557–571
Dehesh K, Bruce WB, Quail PH (1990) A trans-acting factor that binds to a GT-motif in a phytochrome gene promoter. Science 250:1397–1399
Dehesh K, Hung H, Tepperman JM, Quail PH (1992) GT-2: a transcription factor with twin autonomous DNA-binding domains of closely related but different target sequence specificity. EMBO J 11:4131–4144
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Giles KL, Prakash J (eds) (1987) International review of cytology, vol 107. Pollen: cytology and development. Academic Press, Orlando
Gilmartin PM, Memelink J, Hiratsuka K, Kay SA, Chua N-H (1992) Characterization of a gene encoding a DNA binding protein with specificity for a light-responsive element. Plant Cell 4:839–849
Goodspeed TH (1954) The genus Nicotiana: origins, relationships and evolution of its species in light of their distribution, morphology and cytogenetics. Chronica Botanica, Walham
Hamilton DA, Roy M, Rueda J, Sindhu RK, Sanford J, Mascarenhas JP 81992) Dissection of a pollen-specific promoter from maize by transient transformation assays. Plant Mol Biol 18:211–218
Henikoff S (1984) Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28:351–359
Heslop-Harrison J (1987) Pollen germination and pollen-tube growth. In: Giles KL, Prakash J (eds) International review of cytology, vol 107. Pollen: cytology and development. Academic Press, Orlando, pp 1–78
Loenen WAM, Blattner RR (1983) Lambda charon vectors (Ch 32, 33, 34, 35) adapted for DNA cloning in recombinant-deficient hosts. Gene 26:171–179
Mascarenhas JP (1990) Gene activity during pollen development. Annu Rev Plant Phys Mol Biol 41:317–338
McCormick S, Yamaguchi J, Twell D (1991) Deletion analysis of pollen-expressed promoters. In Vitro Cell Dev Biol 27P:15–20
Okamuro JK, Goldberg RB (1985) Tobacco single-copy DNA is highly homologous to sequences present in the genomes of its diploid progenitors. Mol Gen Genet 198:290–298
Ottaviano E, Mulcahy DL, Sari Gorla M, Bergamini Mulcahy G (eds) Angiosperm pollen and ovules. Springer, New York, Berlin, Heidelberg
Reijnen WH, Vanherpen MMA, Degroot PFM, Olmedilla A, Schrauwen JAM, Weterings KAP, Wullems GJ (1991) Cellular localization of a pollen-specific messenger RNA by in situ hybridization and confocal laser scanning microscopy. Sex Plant Reprod 4:254–257
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold spring Harbor laboratory press, Cold Spring Harbor, NY
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schrauwen JAM, Degroot PFM, Vanherpen MMA, Vanderlee T, Reijnen WH, Weterings KAP, Wullems GJ (1990) Stage-related expression of mRNAs during pollen development in lily and tobacco. Planta 182:298–304
Scott R, Dagless E, Hodge R, Paul W, Soufleri I, Draper J (1991a) Patterns of gene expression in developing anthers of Brassica napus. Plant Mol Biol 17:195–207
Scott R, Hodge R, Paul W, Draper J (1991b) The molecular biology of anther differentiation. Plant Sci 80:167–191
Singer RH (1993) Spatial organization of mRNA within cells. J Cell Biochem 52:125–126
Twell D, Klein TM, Fromm ME, McCormick S (1989) Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol 91:1270–1274
Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S (1991) Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev 5:496–507
Vandermeer IM, Stam ME, Vantunen AJ, Mol JNM, Stuitje AR (1992) Antisense inhibition of flavonoid biosynthesis in Petunia anthers results in male sterility. Plant Cell 4:253–262
Weterings K, Reijnen W, Aarssen R van, Kortstee A, Spijkers J, Herpen M van, Schrauwen J, Wullems G (1992) Characterization of a pollen-specific cDNA clone from Nicotiana tabacum expressed during microgametogenesis and germination. Plant Mol Biol 18:1101–1111
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weterings, K., Reijnen, W., Wijn, G. et al. Molecular characterization of the pollen-specific genomic clone NTPg303 and in situ localization of expression. Sexual Plant Reprod 8, 11–17 (1995). https://doi.org/10.1007/BF00228757
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228757