Abstract
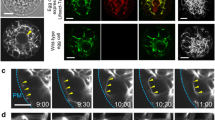
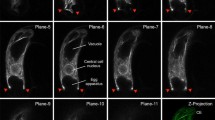
The microtubular and actin cytoskeletons have been investigated during megagametogenesis in Arabidopsis thaliana using immunofluorescence labelling of isolated coenocytic and mature embryo sacs. We found both actin and microtubules (MTs) to occur in abundance throughout megagametogenesis and in all constituent cells of the mature embryo sac. During many stages, the patterns of distribution of these cytoskeletal elements are congruent and may prove to be co-aligned. Many changes in the arrays of MTs and microfilaments take place and indicate varying roles of the cytoskeleton in the different stages and cell types of megagametogenesis. Two major populations of MTs recur throughout embryo sac formation: (1) Elaborate nuclear-based networks are found during the two-nucleate and four-nucleate developmental stages as well as in the egg cell. These arrays may function in positioning the nuclei. (2) Cytoplasmic MTs in longitudinal orientation in the two-nucleate embryo sac, synergids and part of the egg cell, or in a reticulate pattern in the four-nucleate embryo sac, egg and central cell probably participate in organization of the cytoplasm. Synergid MTs converge at the filiform apparatus. Preprophase bands of MTs are absent throughout megagametogenesis but phragmoplast arrays occur during cellularization of the embryo sac. Well developed arrays of cortical MTs are restricted to the antipodal cells. A large concentration of MTs in the part of the egg cell adjacent to the synergids is well placed for being involved with sperm cell movement within the degenerative synergid. On the basis of the morphology of the cytoskeleton, we concur with views that the shape of megagametophyte is largely determined by the surrounding tissues, including the integumentary tapetum.
Similar content being viewed by others
References
Bednara J, Willemse MTM, Lammeren AAM Van (1990) Organization of the actin cytoskeleton during megasporogenesis in Gasteria verrucosa visualized with fluorescent-labelled phalloidin. Acta Bot Neerl 39:43–48
Bhandari NN, Chitralekha P (1989) Cellularization of the female gametophyte in Ranunculus sceleratus. Can J Bot 67:1325–1330
Bloodgood RA (1988) Gliding motility and the dynamics of flagellar membrane glycoproteins in Chlamydomonas reinhardtii. J Protozool 35:552–558
Bloodgood RA (1989) Gliding motility: can regulated protein movements in the plasma membrane drive whole cell locomotion? Cell Motil Cytoskel 14:340–344
Bowman JL (1993) Arabidopsis: an atlas of morphology and development. Springer, New York Berlin Heidelberg (in press)
Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1:37–52
Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79:11–17
Cass DD, Karas I (1974) Ultrastructural organization of the egg of Plumbago zeylanica. Protoplasma 81:49–62
Cass DD, Peteya DJ, Robertson BL (1985) Megagametophyte development in Hordeum vulgare. I. Early megagametogenesis and the nature of cell wall formation. Can J Bot 63:2164–2171
Cass DD, Peteya DJ, Robertson BL (1986) Megagametophyte development in Hordeum vulgare. 2. Later stages of wall development and morphological aspects of megagametophyte cell differentiation. Can J Bot 64:2327–2336
Folsom MW, Cass DD (1989) Embryo sac development in soybean: ultrastructure of megasporogenesis and early megagametogenesis. Can J Bot 67:2841–2849
Folsom MW, Cass, D.D. (1990) Embryo sac development in soybean: cellularization and egg apparatus expansion. Can J Bot 68:2135–2147
Franssen-Verheijen MAW, Willemse MTM (1990) The ovule of Gasteria verrucosa at receptivity of the stigma: an ultrastructural study. Acta Bot Neerl 39:53–63
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 229–292
Gunning BES, Hardham AR (1982) Microtubules. Annu Rev Plant Physiol 33:651–698
Huang B-Q, Russell SD (1992) Female germ unit: organization, reconstruction and isolation. Int Rev Cytol 140:233–293
Huang B-Q, Russell SD, Strout GW, Mao L-J (1990) Organization of isolated embryo sacs and eggs of Plumbago zeylanica (Plumbaginaceae) before and after fertilization. Am J Bot 77:1401–1410
Kadota A, Wada M (1989) Circular arrangement of cortical F-act-in around the subapical region of a tip-growing fern protonemal cell. Plant Cell Physiol 30:1183–1186
Kennell JC, Horner HT (1985) Megasporogenesis and megagametogenesis in soybean, Glycine max. Am J Bot 72:1553–1564
Liu B, Palevitz BA (1992) Organization of cortical microfilaments in dividing root cells. Cell Motil Cytoskel 23:252–264
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, New York
Mansfield SG, Briarty LG, Erni, S. (1991) Early embryogenesis in Arabidopsis thaliana. I. The mature embryo sac. Can J Bot 69:447–460
Mayer U, Torres Ruiz RA, Berleth T, Miséra S, Jürgens G (1991) Mutations affecting body organization in the Arabidopsis embryo. Nature 353:402–407
Meinke DW (1985) Embryo-lethal mutants of Arabidopsis thaliana: analysis of mutants with a wide range of lethal phases. Theor Appl Genet 69:543–552
Meinke DW (1991) Perspectives on genetic analysis of plant embryogenesis. Plant Cell 3:857–866
Menzel D, Schliwa M (1986a) Motility in the siphonous green alga Bryopsis. I. Spatial organization of the cytoskeleton and organelle movements. Eur J Cell Biol 40:275–285
Menzel D, Schliwa M (1986b) Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organized arrays of both microtubules and actin filaments. Eur J Cell Biol 40:286–295
Misra RC (1962) Contribution to the embryology of Arabidopsis thalianum (Gay and Monn.). Agra Univ J Res 11:191–199
Murgia M, Huang B-Q, Tucker SC, Musgrave ME (1993) Embryo sac lacking antipodal cells in Arabidopsis thaliana (Brassicaceae). Am J Bot 80:824–838
Palevitz BA (1987) Actin in the preprophase band of Allium cepa. J Cell Biol 104:1515–1519
Pierson ES, Kengen HMP, Derksen J (1989) Microtubules and actin filaments co-localize in pollen tubes of Nicotiana tabacum L. and Lilium longiflorum Thunb. Protoplasma 150:75–77
Poliakova TF (1964) Development of male and female gametophytes of Arabidopsis thaliana (L.) Heynh. Issledov Genet USSR 2:125–133 (in Russian with English summary)
Robinson-Beers K, Pruitt RE, Gasser CS (1992) Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell 4:1237–1249
Russell SD (1992) Double fertilization. Int Rev Cytol 140:357–388
Russell SD, Rougier M, Dumas C (1990) Organization of the early post-fertilization megagametophyte of Populus deltoides. Ultrastructure and implications for male cytoplasmic transmission. Protoplasma 155:153–165
Schmit A-C, Lambert A-M (1987) Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J Cell Biol 105:2157–2166
Sheldon JM, Hawes C (1988) The actin cytoskeleton during male meiosis in Lilium. Cell Biol Int Rep 12:471–476
Shivanna KR, Johri BM (1985) The angiosperm pollen: structure and function. Wiley Eastern, New Delhi
Staiger CJ, Schliwa M (1987) Actin localization and function in higher plants. Protoplasma 141:1–12
Sumner MJ, Van Caeseele L (1990) The development of the central cell of Brassica campestris prior to fertilization. Can J Bot 68:2553–2563
Tanaka I (1991) Microtubule-determined plastid distribution during microsporogenesis in Lilium longiflorum. J Cell Sci 99:21–31
Theunis CH, Pierson ES, Cresti M (1991) Isolation of male and female gametes in higher plants. Sex Plant Reprod 4:145–154
Tilton VR (1981) Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction. IV. Egg apparatus structure and function. New Phytol 88:505–531
Tilton VR, Lersten NR (1981) Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction. III. Nucellus and megagametophyte. New Phytol 88:477–504
Van Lammeren AAM, Bednara J, Willemse MTM (1989) Organization of the actin cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval visualized with rhodaminephalloidin. Planta 178:531–539
Webb MC (1993) Stages of embryo sac and ovule development. In: Bowman JL (ed) Arabidopsis: an atlas of morphology and development. Springer, New York Berlin Heidelberg (in press)
Webb MC, Gunning BES (1988) The microtubular cytoskeleton during embryo sac development in Arabidopsis thaliana. In: Knox RB, Singh MB, Troiani LF (eds) Pollination '88. School of Botany, University of Melbourne, Melbourne, pp 62–66
Webb MC, Gunning BES (1990) Embryo sac development in Arabidopsis thaliana. I. Megasporogenesis, including the microtubular cytoskeleton. Sex Plant Reprod 3:244–256
Webb MC, Gunning BES (1991) The microtubular cytoskeleton during development of the zygote, proembryo and free-nuclear endosperm in Arabidopsis thaliana (L.) Heynh. Planta 184:187–195
Webb MC, Gunning BES (1992) The cytoskeleton during megagametogenesis, fertilization and proembryo development in Arabidopsis thaliana. In: Proceeding of the XI international symposium — embryology and seed reproduction. Institute of the Academy of Sciences of the USSR, Leningrad pp 611–612
Webb MC, Gunning BES (1993) Cell biology of embryo sac development in Arabidopsis. In: Williams EG, Knox RB, Clarke AE (eds) Genetic control of self-incompatibility and reproductive development in flowering plants. Kluwer Academic Publishers, Dordrecht (in press)
Willemse MTM, Van Lammeren AAM (1988) Structure and function of the microtubular cytoskeleton during megasporogenesis and embryo sac development in Gasteria verrucosa (Mill.) H. Duval. Sex Plant Reprod 1:74–82
Williamson RE (1986) Organelle movements along actin filaments and microtubules. Plant Physiol 82:631–634
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Webb, M.C., Gunning, B.E.S. Embryo sac development in Arabidopsis thaliana II. The cytoskeleton during megagametogenesis. Sexual Plant Reprod 7, 153–163 (1994). https://doi.org/10.1007/BF00228488
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228488