Abstract
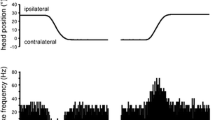
Vestibular nuclei (Vn) neurons and floccular Purkinje (P) cells of unanesthetized paralyzed mice (B6CBA) responding to horizontal angular acceleration in the dark (type I and type II neurons) were studied by extracellular recordings with micropipettes while varying either the frequency (and velocity) or the amplitude (and velocity) of the sinusoidal rotation, keeping the respective third parameter constant. Phase and sensitivity were analyzed by a Fourier analysis and a “best sine fitting” program. Recording sites were localized by means of small iontophoretically applied horseradish peroxidase markings. The neuronal response amplitude at fundamental frequency (determined from peristimulus time histograms) increased with the frequency and amplitude of the sinusoidal rotation for both Vn and floccular neurons (0.05–0.5 Hz; ±10 to ±60° amplitude). Stimulus frequency/response amplitude and stimulus amplitude/response amplitude curves of floccular neurons were distinctly lower in magnitude than those of Vn neurons (P<0.01). Accordingly, the sensitivity (re velocity) curves of Vn neurons and P cells differed in magnitude significantly (decreasing slightly with increasing stimulus frequency and amplitude in Vn neurons and more or less independent of stimulus parameters in floccular P cells). Response amplitudes of type I and type II neurons did not differ from each other. Phase advance relative to head angular velocity in the midfrequency range in Vn neurons was very small, indicating a head velocity signal carried by the Vn neurons. In floccular P cells phase advance was only small at 0.1 Hz (amplitude ±35°), but increased with augmenting frequency to 140° at 0.5 Hz. With a constant stimulus frequency (0.3 Hz) and varied stimulus amplitude, phase advance was 90° at ±20° amplitude and 60° at ±60° amplitude. Data are shown for the first time in which both the stimulus frequency and the stimulus amplitude have been varied in the same species and in the same neuron. The results demonstrate that the single data are in general well within the range of those found in other species, but they demonstrate further that phase behavior is dependent on the stimulus paradigm. The data provide the basis for comparative studies with mutant mice.
Similar content being viewed by others
References
Angaut P, Brodal A (1967) The projection of the “vestibulocerebellum” onto the vestibular nuclei in the cat. Arch Ital Biol 105:441–479
Blanks RHI, Precht W (1976) Functional characterization of primary vestibular afferents in the frog. Exp Brain Res 25:369–390
Blanks RHI, Precht W (1983) Responses of units in the rat cerebellar flocculus during optokinetic and vestibular stimulation. Exp Brain Res 53:1–15
Blanks RHI, Precht W, Giretti ML (1977) Response characteristics and vestibular receptor convergence of frog cerebellar Purkinje cell. A natural stimulation study. Exp Brain Res 27:181–201
Buettner UW, Büttner U, Henn V (1978) Transfer characteristics of neurons in vestibular neuclei of the alert monkey. J Neurophysiol 41:1614
Cazin L, Precht W, Lannou J (1980) Optokinetic responses of vestibular nucleus neurons in the rat. Pflugers Arch 384:31–38
Duensing F, Schäfer KP (1958) Die Aktivität einzelner Neurone im Bereich der Vestibulariskerne bei Horizontalbeschleunigunen unter besonderer Berücksichtigung des vestibulären Nystagmus. Arch Psychiatr Nervenkr 198:225–252
Fernandez C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34:661–675
Ghelarducci B, Ito M, Yagi N (1975) Impulse discharges from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Res 87:66–72
Grüsser-Cornehls U (1983) Response of vestibular neurons from the nucleus vestibularis and the flocculus in wildtype mice and mutants. Soc Neurosci Abstr 9(1):524
Grüsser-Cornehls U (1988) Compensatory mechanisms at the level of the vestibular nuclei following post-natal degeneration of specific cerebellar cell classes and ablation of the cerebellum in mutant mice. In: Flohr H (eds) Post-lesion neural plasticity. Springer, Berlin Heidelberg New York, pp 431–442
Grüsser-Cornehls U (1995) Responses of flocculus and vestibular nuclei neurons in Weaver mutant mice (B6CBA wv/wv) to combined head and body rotation. Exp Brain Res 107:26–33
Grüsser-Cornehls U, Plassmann W, Helmchen Ch, Kahle G (1986) Single unit activity in the vestibular nuclei of normal mice and mutant mice with genetic cerebellar defects (abstract). Dev Oculomot Res. IUPS Satellite Meeting, Gleneden Beach, Oregon, p 11
Grüsser-Cornehls U, Luy M, Bäurle J (1995) Electrophysiology and GABA-immunocytochemistry in the vestibular nuclei of normal (C57BL/6J) and Leaner mutant mice. Brain Res (in press)
Herrick CJ (1924) Origin and evolution of the cerebellum. Arch Neurol Psychiatr 11:621–652
Larsell O (1923) The cerebellum of the frog. J Comp Neurol 36:89–112
Larsell O (1929) The comparative morphology of the membraneous labyrinth and the lateral-line organs in their relation to the development of the cerebellum. In: The cerebellum. William and Wilkins, Baltimore, pp 279–328
Leonard CS, Simpson JI (1983) Rotational polarity of Purkinje cell activity in the rabbit flocculus. Soc Neurosci Abstr 9 (1):608
Linder H (1951) Statistische Methoden für Naturwissenschaftler, Mediziner and Ingenieure. Birkhäuser, Basel
Lisberger SG, Fuchs AF (1974) Response of flocculus Purkinje cells to adequate vestibular stimulation in the alert monkey: fixation vs. compensatory eye movements. Brain Res 69:347–353
Lisberger SG, Fuchs AF (1978a) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smoothpursuit eye movements and passive head rotation. J Neurophysiol 41:733–763
Lisberger SG, Fuchs AF (1978b) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. II. Mossy fiber firing patterns during horizontal head rotation and eye movement. J Neurophysiol 41:764–777
Maekawa K, Simpson JI (1973) Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol 36:649–666
Maekawa K, Takeda T (1975) Mossy fiber responses evoked in the cerebellar flocculus of rabbits by stimulation of the optic pathway. Brain Res 98:590–595
Melvill-Jones G, Milsum JH (1970) Characteristics of neural transmission from the semicircular canal to the vestibular nuclei of cats. I Physiol (Lond) 209:295–316
Miyashita Y (1979) Interaction of visual and canal inputs on the oculomotor system via the cerebellar flocculus. Prog Brain res 50:695–702
Rupp G, Grüsser-Cornehls U (1990) Responses of vestibularly activated neurons in the nucleus praepositus hypoglossi of wildtype and Weaver mutant mice. In: Elsner N, Roth T (eds) Brain-perception-cognition. Proceedings of the 18th Göttingen Neurobiology Conference, Stuttgart. Thieme, New York, p 174
Schneider LW, Anderson DJ (1976) Transfer characteristics of first and second order lateral canal vestibular neurons in gerbil. Brain Res 112:61–76
Shinoda Y, Yoshida K (1974) Dynamic characteristics of responses to horizontal head angular acceleration in vestibulo-ocular pathway in the cat. J Neurophysiol 37. 653–673
Stone LS, Lisberger SG (1990) Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol 63:1241–1261
Waespe W, Kenn V (1981) Visual-vestibular interaction in the flocculus of the alert monkey. II. Purkinje cell activity. Exp Brain Res 43:349–360
Waespe W, Rudinger D, Wolfensberger M (1985) Purkinje cell activity in the flocculus of vestibular neurectomized and normal monkeys during optokinetic nystagmus (OKN) and smooth pursuit eye movements. Exp Brain Res 60:243–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grüsser-Cornehls, U., Niemschynski, A. & Plassmann, W. Vestibular responses of flocculus and vestibular nuclei neurons in mice (B6CBA). Exp Brain Res 107, 17–25 (1995). https://doi.org/10.1007/BF00228012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228012