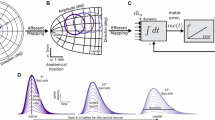
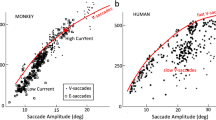
Abstract
The synaptic organization of the saccade-related neuronal circuit between the superior colliculus (SC) and the brainstem saccade generator was examined in an awake monkey using a saccadic, midflight electrical-stimulation method. When microstimulation (50–100 μA, single pulse) was applied to the SC during a saccade, a small, conjugate contraversive eye movement was evoked with latencies much shorter than those obtained by conventional stimulation. Our results may be explained by the tonic inhibition of premotor burst neurons (BNs) by omnipause neurons that ceases during saccades to allow BNs to burst. Thus, during saccades, signals originating from the SC can be transmitted to motoneurons and seen in the saccade trajectory. Based on this hypothesis, we estimated the number of synapses intervening between the SC and motoneurons by applying midflight stimulation to the SC, the BN area, and the abducens nucleus. Eye position signals were electronically differentiated to produce eye velocity to aid in detecting small changes. The mean latencies of the stimulus-evoked eye movements were: 7.9±1.0 ms (SD; ipsilateral eye) and 7.8±0.9 ms (SD; contralateral eye) for SC stimulation; 4.8±0.5 ms (SD; ipsilateral eye) and 5.1±0.7 ms (SD; contralateral eye) for BN stimulation; and 3.6±0.4 ms (SD; ipsilateral eye) and 5.2±0.8 ms (SD; contralateral eye) for abducens nucleus stimulation. The time difference between SC- and BN-evoked eye movements (about 3 ms) was consistent with a disynaptic connection from the SC to the premotor BNs.
Similar content being viewed by others
References
Baker RG, Mano N, Shimazu H (1969) Intracellular recording of antidromic responses form abducens motoneurons in the cat. Brain Res 15: 573–576
Büttner-Ennever JA, Cohen B, Pause M, Fries W (1988) Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J Comp Neurol 267: 307–321
Chimoto S, Iwamoto Y, Shimazu H, Yoshida K (1996) Monosynaptic activation of medium-lead burst neurons from the superior colliculus in the alert cat. J Neurophysiol 75: 2658–2661
Cohen B, Komatsuzaki A (1972) Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol 36: 101–117
Curthoys IS, Markham CH, Furuya N (1984) Direct projection of pause neurons to nystagmus-related excitatory burst neurons in the cat pontine reticular formation. Exp Neurol 83: 414–422
Evinger C, Kaneko CRS, Fuchs AF (1982) Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J Neurophysiol 47: 827–844
Fetz EE, Cheney PD, German DC (1976) Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res 114: 505–510
Grantyn A, Berthoz A (1977) Synaptic actions of the superior colliculus on medial rectus motoneurons in the cat. Neuroscience 2: 945–951
Grantyn AA, Grantyn R (1976) Synaptic actions of tectofugal pathways on abducens motoneurons in the cat. Brain Res 105: 269–285
Grantyn A, Grantyn R (1982) Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res 46: 243–256
Guitton D, Munoz D (1991) Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. I. Identification, localization and effects of behavior on sensory responses. J Neurophysiol 66: 1605–1623
Hepp K, Henn V (1983) Spatio-temporal receding of rapid eye movement signals in the monkey paramedian pontine reticular formation (PPRF). Exp Brain Res 52: 105–120
Hepp K, Henn V, Vilis T, Cohen B (1989) Brainstem regions related to saccadic generation. In: Wurtz RH, Goldberg ME (eds) The neurobiology of saccadic eye movements. Elsevier, Amsterdam, pp 105–212
Highstein SM, Baker R (1978) Excitatory termination of abducens internuclear neurons on medial rectus motoneurons: relationship to syndrome of internuclear opthalmoplegia. J Neurophysiol 41: 1647–1661
Hikosaka O, Kawakami T (1977) Inhibitory reticular neurons related to the quick phase of vestibular nystagmus — their location and projection. Exp Brain Res 27: 377–396
Hikosaka O, Sakamoto M, Usui S (1989) Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol 61: 780–798
Hikosaka O, Wurtz RH (1985a) Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in the monkey superior colliculus. J Neurophysiol 53: 266–291
Hikosaka O, Wurtz RH (1985b) Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in the monkey substantia nigra pars reticulata. J Neurophysiol 53: 292–308
Igusa Y, Sasaki S, Shimazu H (1980) Excitatory premotor burst neurons in the cat pontine reticular formation related to the quick phase of vestibular nystagmus. Brain Res 82: 451–456
Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538
Keller EL (1974) Participation of the medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol 37: 316–332
Kori A, Miyashita N, Kato M, Hikosaka O, Usui S, Matsumura M (1995) Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J Neurosci 15: 928–941
Matsumura M, Kojima J, Gardiner TW, Hikosaka O (1992) Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol 67: 1615–1632
Moschovakis AK, Karabelas AB (1985) Observations on the somatodendritic morphology and axonal trajectory of intracellularly HRP-labeled efferent neurons located in the deep layers of the superior colliculus of the cat. J Comp Neurol 239: 276–308
Moschovakis AK, Karabelas AB, Highstein SM (1988) Structure-function relationships in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol 60: 263–302
Munoz DP, Wurtz RH (1993a) Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575
Munoz DP, Wurtz RH (1993b) Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70: 576–589
Munoz DP, Guitton D, Pélisson D (1991) Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol 66: 1642–1666
Nakao S, Sasaki S (1980) Excitatory inputs from interneurons in the abducens nucleus to medial rectus motoneurons mediating conjugate horizontal nystagmus in the cat. Exp Brain Res 39: 283–293
Nakao S, Curthoys IS, Markham CH (1980) Direct inhibitory projection of pause neurons to nystagmus-related pontomedullary reticular burst neurons in the cat. Exp Brain Res 40: 283–293
Precht W, Schwindt PC, Magherini PC (1974) Tectal influences on cat ocular motoneurons. Brain Res 82: 27–40
Raybourn MS, Keller EL (1977) Colliculoreticular organization in primate oculomotor system. J Neurophysiol 40: 861–878
Robinson DA (1972) Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808
Sasaki S, Shimazu H (1981) Reticulovestibular organization participating in generation of horizontal fast eye movement. Ann NY Acad Sci 374: 130–143
Schiller PH, Koerner F (1971) Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol 34: 920–936
Schiller PH, Stryker M (1972) Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35: 915–924
Scudder CA, Fuchs AF, Langer TP (1988) Characterization and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol 59: 1430–1454
Smit AC, Van Gisbergen JAM, Cools AR (1987) A parametric analysis of human saccades in different experimental paradigms. Vision Res 27: 1745–1762
Sparks DL, Hartwich-Young R (1989) The deep layers of the superior colliculus. In: Wurtz RH, Goldberg ME (eds) The neurobiology of saccadic eye movements. Elsevier, Amsterdam, pp 213–255
Sparks DL, Holland R, Guthrie BL (1976) Size and distribution of movement fields in the monkey superior colliculus. Brain Res 113: 21–34
Strassman A, Highstein SM, McCrea RA (1986a) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249: 337–357
Strassman A, Highstein SM, McCrea RA (1986b) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249: 358–380
Strassman A, Evinger C, McCrea RA, Baker RG, Highstein SM (1987) Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp Brain Res 67: 436–440
Suzuki H, Azuma M (1976) A glass insulated “Elgiloy” microelectrode for recording unit activity in chronic monkey experiments. Electroencephalogr Clin Neurophysiol 41: 93–95
Wurtz RH, Goldberg ME (1971) Superior colliculus cell responses related to eye movements in awake monkeys. Science 171: 82–84
Wurtz RH, Goldberg ME (1972) Activity of superior colliculus in behaving monkey. III. Cells discharging before eye movements. J Neurophysiol 35: 575–586
Yoshida K, McCrea R, Berthoz A, Vidal PP (1982) Morphological and physiological characteristics of inhibitory burst neurons controlling horizontal rapid eye movements in the alert cat. J Neurophysiol 48: 761–784
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyashita, N., Hikosaka, O. Minimal synaptic delay in the saccadic output pathway of the superior colliculus studied in awake monkey. Exp Brain Res 112, 187–196 (1996). https://doi.org/10.1007/BF00227637
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227637