Summary
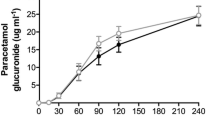
CCK is known to be a major endocrine stimulant of the exocrine pancreas. However, the influence of various compositions of nutrients and their mode of administration on plasma CCK is still not clear. We therefore studied plasma CCK after either oral or intraduodenal administration of various solid and liquid diets. Plasma CCK was measured by bioassay. Nine healthy male volunteers received three different isocaloric diets via intraduodenal perfusion (1 kcal/ml; 300 ml/90 min). Diet A consisted of low-molecular oligopeptides (energy: protein 18%, fat 22%, carbohydrates 60%); diet B was a high molecular diet enriched with fat and fiber (protein 15%, fat 30%, carbohydrates 55%, fiber 1 g/100 ml); and diet C was a high molecular diet, poor in fat and fiber-free (protein 14%, fat 7%, carbohydrates 79%). All diets caused a rapid increase in plasma CCK, followed by a slow decrease. The highest CCK plasma values were achieved with diet B (8.4±1.6 pM); diets A and C led to similar, rather low plasma CCK values (A: 5.0±0.7 pM). Another five volunteers received the above-described liquid diets orally. Integrated CCK plasma values were similar for all oral liquid diets studied and not different from those seen after intraduodenal administration of the high-molecular high-fat diet B. Oral administration of a solid diet (ten volunteers) comparable to diet B in calories and nutrient composition caused a rather small and delayed increase in plasma CCK. Surprisingly, oral administration of a diet very rich in calories, especially fat, which caused complete satiety (spaghetti with ground beef, lettuce, banana; 1713 kcal, fat 34%, protein 16%, carbohydrates 50%; seven volunteers) resulted in maximum CCK values of only 7.3±1.2 pM. We conclude that, despite the significant differences in plasma CCK in relation to nutrient composition and mode of administration, all of the diets tested caused significant rises in plasma CCK.
Similar content being viewed by others
Abbreviations
- CCK:
-
cholecystokinin
- TFA:
-
trifluoroacetic acid
References
Bradford M (1976) A rapid and sensitive method for the quantitation of migrogram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Byrnes DJ, Henderson L, Borody T, Rehfeld JF (1981) Radioimmunoassay of cholecystokinin in human plasma. Clin Chim Acta 111: 81–89
Calam J, Ellis A, Dockray GJ (1982) Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest 69:218–225
Cantor P (1986) Evaluation of a radioimmunoassay for cholecystokinin in human plasma. Scand J Clin Lab Invest 46:213–221
Cantor P (1989) Cholecystokinin in plasma. Digestion 42:181–201
Cantor P, Petronijevic L, Pedersen JF, Worning H (1986) Cholecystokinetic and pancreozymic effect of O-sulfated gastrin compared with nonsulfated gastrin and cholecystokinin. Gastroenterology 91:1154–1163
Eysselein VE, Reeve JR Jr, Shively JE, Miller C, Walsh JH (1984) Isolation of a large cholecystokinin precursor from canine brain. Proc Natl Acad Sci USA 81:6565–6568
Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR Jr (1987) Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem 262:214–217
Eysselein VE, Eberlein G, Ho FJ, Goebell H, Reeve JR Jr (1988) An amino-terminal fragment of cholecystokinin-58 is present in the gut: evidence for a similar processing site of procholecystokinin in canine gut and brain. Regul Pept 22:205–215
Harper AA, Raper HS (1943) Pancreozymin, a stimulant of secretion of pancreatic enzymes in extracts of the small intestine. J Physiol 102:115–125
Hildebrand P, Beglinger C, Gyr K, Jansen JBMJ, Rovati LC, Zuercher M, Lamers CBHW, Setnikar I, Stalder GA (1990) Effects of a cholecystokinin receptor antagonist on intestinal phase of pancreatic and biliary responses in man. J Clin Invest 85:640–646
Ivy AC, Oldberg E (1928) A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol 65:599–613
Jung DH (1980) Preparation and application of procion yellow starch for amylase assay. Clin Chim Acta 100:97–100
Kellow JE, Miller LJ, Phillips SF, Haddad AC, Zinsmeister AR, Charboneau JW (1987) Sensitivities of human jejunum, ileum, proximal colon, and gallbladder to cholecystokinin octapeptide. Am J Physiol 252: G345–346
Koretz RL, Meyer JH (1980) Elemental diets — facts and fantasies. Gastroenterology 78:393–410
Liddle RA, Goldfine ID, Williams JA (1984) Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87:542–549
Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA (1985) Cholecystokinin bioactivity in human plasma. J Clin Invest 75:1144–1152
Liddle RA, Morita ET, Conrad CK, Williams JA (1986) Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest 77:992–996
Maton PN, Selden AC, Chadwick VS (1982) Large and small forms of cholecystokinin in human plasma: measurement using high pressure liquid chromatography and radioimmunoassay. Regul Pept 4:251–260
Mössner J, Regner UF, Zeeh JM, Bruch H-P, Eberlein G (1989) Influence of food on plasma cholecystokinin and gastrin in patients with partial gastric resections and Roux-en-Y anastomosis. Z Gastroenterol 27:94–98
Mössner J, Zeeh JM, Eberlein G, Schäffer M, Regner U, Grandt D, Goebell H, Eysselein VE (1991) Determination of various molecular forms of cholecystokinin from canine mucosa by radioimmunoassay and bioassay. Digestion 48:210–219
Reeve JR Jr, Eysselein VE, Walsh JH, Ben-Avram CM, Shively JE (1986) New molecular forms of cholecystokinin. J Biol Chem 261:16392–16397
Rehfeld JF (1984) How to measure cholecystokinin in plasma? Gastroenterology 87:434–438
Williams JA, Korc M, Dormer RL (1978) Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol 235:E517–524
Williams JA (1982) Cholecystokinin: a hormone and a neurotransmitter. Biomed Res 3:107–121
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mössner, J., Grumann, M., Zeeh, J. et al. Influence of various nutrients and their mode of application on plasma cholecystokinin (CCK) bioactivity. Clin Investig 70, 125–129 (1992). https://doi.org/10.1007/BF00227353
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227353