Summary
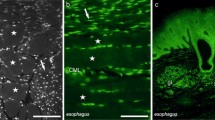
The guinea-pig intestine was found to harbor nerve fibers containing immunoreactive cholecystokinin (CCK), gastrin-releasing peptide (GRP), neurotensin or β- endorphin. Such fibers occurred in the myenteric and submucous ganglia and in the smooth muscle. GRP- and CCK-fibers, in addition, were found in the mucosa. Following colchicine treatment, neuronal perikarya in the myenteric ganglia displayed CCK-, GRP-, or β-endorphin immunoreactivity. CCK-immunoreactive perikarya were located also in the submucous ganglia. Neurotensin-immunoreactive cell bodies could not be detected. The presence of immunoreactive neuronal perikarya in intramural ganglia indicates that CCK-, GRP- and β-endorphin-containing fibers are intrinsic to the gut wall. GRP, neurotensin, and β-endorphin were identified in extracts of smooth muscle by immunochemical and Chromatographic analysis.
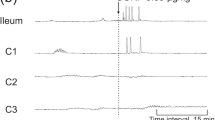
CCK-8, GRP and neurotensin contracted the isolated taenia coli. Tetrodotoxin reduced the response to CCK-8 but not that to GRP and neurotensin, suggesting that the two latter peptides act directly on smooth muscle receptors. The effect of CCK-8 is partly mediated by cholinergic nerves, since not only tetrodotoxin but also atropine greatly reduced the CCK-8-induced contractile response. The substance P (SP) antagonist, (d-Pro2, d-Trp7,9)-SP1–11 had no effect on the CCK-8-induced contraction of the taenia. CCK-8 enhanced the SP-mediated (atropine-resistant) contractile response to electrical stimulation but not that mediated by acetylcholine. β-Endorphin had no effect on the tension of the muscle but reduced the response to electrical stimulation (cholinergic as well as SP-mediated) through a naloxone-sensitive mechanism.
While CCK-8 and β-endorphin seem to play neuromodulatory roles in the taenia coli, the significance of GRP and neurotensin remains enigmatic.
Similar content being viewed by others
References
Ambache N, Freeman MA (1968) Atropine-resistant longitudinal muscle spasm due to excitation of non-cholinergic neurones in Auerbach's plexus. J Physiol 199:705–727
Barthó L, Holzer P, Lembeck F (1983) Sympathetic control of substance P releasing enteric neurones in the guinea pig ileum. Neurosci Lett 38:291–296
Bramnert M, Ekman R, Larsson I, Thorell J (1982) Characterization and application of a radioimmunoassay for β-endorphin using an antiserum with negligible cross-reactivity against β-lipotropin. Reg Peptides 5:65–75
Buffa R, Solovieva I, Fiocca R, Giorgino S, Rindi G, Solcia E, Mochizuchi T, Yanaihara C, Yanaihara N (1982) Localization of bombesin and GRP (gastrin releasing peptide) sequences in gut nerves or endocrine cells. Histochemistry 76:457–467
Burnstock G, Campbell G, Rand MJ (1966) The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol 182:504–526
Carraway RE, Leeman SE (1976a) Radioimmunoassay for neurotensin, a hypothalamic peptide. J Biol Chem 251:7035–7044
Carraway R, Leeman SE (1976b) Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine and stomach. J Biol Chem 251:7045–7052
Coons AH, Leduc EH, Connolly JM (1955) Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med 102:49–60
Costa M, Buffa R, Furness JB, Solda E (1980a) Immunohistochemical localization of polypeptides in peripheral autonomic nerves using whole mount preparations. Histochemistry 65:157–165
Costa M, Furness JB, Buffa R, Said SI (1980b) Distribution of enteric nerve cell bodies and axons showing immunoreactivity for vasoactive intestinal polypeptide in the guinea-pig intestine. Neuroscience 5:587–596
Del Tacca M, Soldani G, Crema A (1970) Experiments on the mechanism of action of caerulein at the level of the guinea-pig ileum and colon. Agents Actions 1:176–182
Dockray GJ, Hutchison JB (1980) Cholecystokinin octapeptide in guinea-pig ileum myenteric plexus: localization and biological action. J Physiol (Lond) 300:28–29
Dockray GJ, Vaillant C, Walsh JH (1979) The neuronal origin of bombesin-like immunoreactivity in the rat gastrointestinal tract. Neuroscience 4:1561–1568
Dockray GJ, Vaillant C, Hutchison JB (1981) Immunochemical characterization of peptides in endocrine cells and nerves with particular reference to gastrin and cholecystokinin. In: Grossman MI, Brazier MAB, Lechago J (eds) Cellular basis of chemical messengers in the digestive system. Academic Press New York, pp 215–230
Erspamer V (1980) Peptides of the amphibian skin active on the gut. II. Bombesin-like peptides: Isolation, structure, and basic functions. In: Glass GBJ (ed) Gastrointestinal hormones. Raven Press, New York, pp 343–362
Erspamer V, Erspamer GF, Inselvini M, Negri (1972) Occurrence of bombesin and alytesin in extracts of the skin of three European discoglossid frogs and pharmacological actions of bombesin on extravascular smooth muscle. Brit J Pharmacol 45:333–348
Fahrenkrug J (1981) VIP as a neurotransmitter in the peripheral nervous system. In: Said SI (ed) Vasoactive intestinal peptide, Raven Press, New York, pp 361–372
Furness JB, Costa M (1973) The nervous release and the action of substances which affect intestinal muscle through neither adrenoceptors nor cholinoceptors. Phil Trans R Soc Lond (Biol) 265:123–133
Furness JB, Costa M (1979) Projections of intestinal neurons showing immunoreactivity for vasoactive intestinal polypeptide are consistent with these neurons being the enteric inhibitory neurons. Neurosci Lett 15:199–204
Furness JB, Costa M (1980) Types of nerves in the enteric nervous system. Neuroscience 5:1–20
Furness JB, Costa M (1981) Enteric inhibitory nerves and VIP. In: Said SI (ed) Vasoactive intestinal peptide, Raven Press, New York, pp 391–406
Ghatei MA, Jung RT, Stevenson JC, Hillyard CJ, Adrian TE, Lee YC, Christofides ND, Sarson DL, Mashiter K, MacIntyre I, Bloom SR (1982) Bombesin: Action on gut hormones and calcium in man. J Clin Endocrinol Metab 54:980–985
Håkanson R, Leander S, Sundler F, Uddman R (1981a) P-type nerves: Purinergic or peptidergic. In: Lechago J, Grossman MI (eds) Cellular basis of chemical messengers in the digestive system. UCLA Forum in Medical Sciences, pp 169–199
Håkanson R, Sundler F, Uddman R (1981b) Distribution and topography of peripheral VIP nerve fibers: Functional implications. In: Said SI (ed) Vasoactive intestinal peptide, Raven Press, New York, pp 121–144
Håkanson R, Hörig J, Leander S (1982) The mechanism of action of a substance P antagonist (d-Pro2, d-Trp7,9)-SP. Br J Pharmac 77:697–700
Hedner P, Rorsman G (1968) Structures essential for the effect of cholecystokinin on the guinea pig small intestine in vitro. Acta Physiol Scand 74:58–68
Hedqvist P, von Euler US (1975) Influence of substance P on the response of guinea pig ileum to transmural nerve stimulation. Acta Physiol Scand 95:341–343
Hökfelt T, Johansson O, Ljungdahl Å, Lundberg JM, Schultzberg M (1980) Peptidergic neurones. Nature 284:515–521
Hunter WM, Greenwood FC (1962) Preparation of iodine 131-labeled human growth hormone of high specific activity. Nature 194:495–496
Kao CY (1966) Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev 18:997–1049
Kitabgi P, Freychet P (1978) Effects of neurotensin on isolated smooth muscles. Eur J Pharmacol 50:349–357
Kitabgi P, Freychet P (1979) Neurotensin contracts the guinea-pig longitudinal ileal smooth muscle by inducing acetylcholine release. Eur J Pharmacol 56:403–406
Kitabgi P, Vincent J-P (1981) Neurotensin is a potent inhibitor of guinea-pig colon contractile activity. Eur J Pharmacol 74:311–318
Larsson L-I, Rehfeld JF (1979) Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res 165:201–218
Leander S, Håkanson R, Rosell S, Folkers K, Sundler F, Tornqvist K (1981a) A specific substance P antagonist blocks smooth muscle contractions induced by non-cholinergic, non-adrenergic nerve stimulation. Nature 294:467–469
Leander S, Håkanson R, Sundler F (1981b) Nerves containing substance P, vasoactive intestinal polypeptide, enkephalin or somatostatin in the guinea-pig taenia coli. Cell Tissue Res 215:21–39
Lorén I, Aluments J, Håkanson R, Sundler F (1979a) Immunoreactive pancreatic polypeptide (PP) occurs in the central and peripheral nervous system: Preliminary immunocytochemical observations. Cell Tissue Res 200:179–186
Lorén I, Alumets J, Håkanson R, Sundler F (1979b) Distribution of gastrin and CCK-like peptides in rat brain. Histochemistry 59:249–257
McDonald TJ, Jörnvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V (1979) Characterization of a gastrin releasing peptide from non-antral gastric tissue. Biochem Biophys Res Comm 90:227–233
Moghimzadeh E, Ekman R, Håkanson R, Yanaihara N, Sundler F (1983) Neuronal gastrin-releasing peptide in the mammalian gut and pancreas. Neuroscience 10:553–563
Monier S, Kitabgi P (1981) Effects of β-endorphin, met-enkephalin and somatostatin on the neurotensin-induced neurogenic contraction in the guinea-pig ileum. Regul Peptides 2:31–42
Nilsson S, Leander S, Vallgren S, Håkanson R (1983) Gastrins and cholecystokinins release acetylcholine but not substance P from neurons in the guinea-pig taenia coli. Eur J Pharmacol 90:245–250
Polak JM, Bloom SR (1978) Peptidergic nerves of the gastrointestinal tract. Invest Cell Pathol 1:301–326
Rehfeld JF (1978) Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and the small intestine of man and hog. J Biol Chem 253:4022–4027
Rehfeld JF, Golterman N, Larsson L-I, Emson PM, Lee CM (1979) Gastrin and cholecystokinin in central and peripheral neurons. Fed Proc 38:2325–2329
Rökaeus Å, McDonald T (1980) Release of neurotensin-like immunoreactivity (NTLI) from rat intestine following administration of bombesin. Acta Physiol Scand 110:326
Said SI (1980) Peptides common to the nervous system and the gastrointestinal tract. In: Martini L, Ganong WF (eds) Frontiers in neuroendocrinology, vol 6. Raven Press, New York, pp 293–331
Schultzberg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, Elde R, Goldstein M, Said S (1980) Distribution of peptideand catecholamine-containing neurons in the gastrointestinal tract of rat and guinea-pig: Immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine-β-hydroxylase. Neuroscience 5:689–744
Sternberger LA (1979) Immunocytochemistry. 2nd ed. John Wiley & Sons, New York Toronto
Sundler F, Håkanson R, Hammer RA, Alumets J, Carraway R, Leeman SE, Zimmerman EA (1977) Immunohistochemical localization of neurotensin in endocrine cells of the gut. Cell Tissue Res 178:313–321
Sundler F, Håkanson R, Leander S (1980) Peptidergic nervous systems in the gut. Clin Gastroenterol 9:517–543
Sundler F, Håkanson R, Leander S, Uddman R (1982) Light and electron microscopic localization of neurotensin in the gastrointestinal tract. In: Nemeroff CB, Prange AJ (eds) Neurotensin, a brain and gastrointestinal peptide. Ann NY Acad Sci 400:94–103
Tager HS (1976) Coupling of peptides to albumin with difluorodinitrobenzene. Anal Biochem 71:367–375
Vizi ES (1973) Acetylcholine release from guinea-pig ileum by parasympathetic ganglion stimulants and gastrin-like polypeptides. Brit. J Pharmacol 47:765–777
Vizi ES, Bertaccini G, Impicciatore M, Knoll J (1972) Acetylcholine-releasing effect of gastrin and related polypeptides. Eur J Pharmacol 17:175–178
Vuolteenaho O, Vakkuri O, Leppäluoto J (1980) Wide distribution of β-endorphin-like immunoreactivity in extrapituitary tissues of rat. Life Sci 27:57–65
Waterfield AA, Smokcum RWJ, Hughes J, Kosterlitz HW, Henderson G (1977) In vitro pharmacology of the opioid peptides, enkephalins and endorphins. Eur J Pharmacol 43:107–116
Yanaihara N, Yanaihara C, Mochizuki T, Iwahara K, Fujita T, Iwanaga T (1981) Immunoreactive GRP. Peptides (Suppl 2) 2:185–192
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leander, S., Ekman, R., Uddman, R. et al. Neuronal cholecystokinin, gastrin-releasing peptide, neurotensin, and β-endorphin in the intestine of the guinea pig. Cell Tissue Res. 235, 521–531 (1984). https://doi.org/10.1007/BF00226949
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226949