Summary
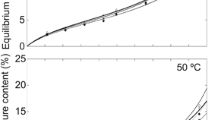
Isosters for Western white pine were experimentally determined at temperatures of 30, 45, 60 and 70°C and relative humidities between 10 and 90%, approximately. The enthalpyentropy and free energy changes for water, were calculated and analyzed. The enthalpy-entropy compensation was proven to exist and the isokinetic temperature was calculated.
Similar content being viewed by others
References
Aguerre, R. J.; Suarez, C.; Viollaz, P. E. 1986: Enthalpy-entropy compensation in sorption phenomena: Application to the prediction of the effect of temperature on food isotherms. J. Food Sci. 51: 1547–1549
Bell, R. P. 1937: Relations between the energy and entropy of solution and their significant. Trans. Faraday Soc. 33: 496–501
Everett, D. H. 1950a: The thermodynamics of adsorption. Part II. Thermodynamics of monolayers on solids. Trans. Faraday Soc. 46: 942–957
Everett, D. H. 1950b: The thermodynamics of adsorption. Part III. Analysis and discussion of experimental data. Trans. Faraday Soc. 46: 957–969
Fontan, F. C.; Chirife, J.; Sancho, E.; Inglesias, H. A. 1982: Analysis of a model for water sorption phenomena in food. J. Food Sci. 47: 1590–1594
Kelsey, K. E.; Clarke, L. N. 1956: The heat of sorption of water by wood. Aust. J. Appl. Sci. 8: 42–45
Krug, R. R.; Hunter, W. G.; Grieger, R. A. 1976: Enthalpy-entropy compensation. — Part 1: Some fundamental statistical problems associated with the analysis of Van't Hoff and Arrhenius data. J. Phys. Chem. 80: 2335–2341
Labuza, T. P. 1980: Enthalpy-entropy compensation in food reactions. Food Tech. 34: 67–77
Leffler, J. E. 1955: The enthalpy-entropy relationship and its implications for organic chemistry. J. Org. Chem. 20: 1202–1231
Lamry, R.; Eyring, H. 1954: Conformation changes in proteins. J. Phys. Chem. 58: 110–120
Nelson, R. M. 1983: A model for sorption of water vapor by cellulosic materials. Wood Fiber Sci. 15 (1): 8–22
Skaar, C. 1989: Wood-water relations. Berlin, Heidelberg, New York, London, Paris, Tokyo: Springer Verlag
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Avramidis, S. Enthalpy-entropy compensation and thermodynamic considerations in sorption phenomena. Wood Sci.Technol. 26, 329–333 (1992). https://doi.org/10.1007/BF00226074
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00226074