Summary
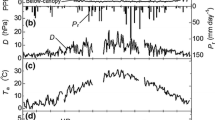
Diurnal courses of stomatal conductance, leaf water potential, and the components of tissue water potential were measured in six canopy species in an elfin cloud forest. High values of stomatal conductance were measured on cloudy days and during early morning and late afternoon of sunny days. Decreases in stomatal conductance with increases in vapour pressure deficit may have been a response to avoid further water deficits and suggested a stomatal response to changes in relative humidity. Daily transpiration varied between 470 and 1014 g m-2 day-1 during cloudy days and between 532 and 944 g m-2 day-1 during clear days. Stomatal conductance may have also responded to changes in leaf water potential, which was minimum at noon. The minimum tissue water potential measured in the field was -1.8 MPa in Myrcianthes fragrans, and the minimum turgor pressure was 0.49 MPa also in M. fragrans. There was a correlation between the osmotic potential and the minimum tissue water potential, suggesting that osmotic potential plays a major role in the maintenance of turgor in these species, in spite of the great variability in the elastic properties of leaf tissues. Turgor pressure decreased during the day following the course of water potential but never approached the turgor loss point, as it has been measured in some lowland rain forest trees. This is a strong indication that elfin cloud forest trees do not suffer severe water deficits, and that small tree stature is not directly related to water shortage.
Similar content being viewed by others
References
Aylett GP (1985) Irradiance, interception, leaf conductance and photosynthesis in Jamaican upper montane rain forest trees. Photosynthetica 19: 323–337
Baynton HW (1968) The ecology of an elfin forest in Puerto Rico, 2. The microclimate of Pico del Oeste. J. Arnold Arbor Harv Univ 49: 419–430
Baynton HW (1969) The ecology of an elfin forest in Puerto Rico, 3. Hilltop and forest influence on the microclimate of Pico del Oeste. J Arnold Arbor Harv Univ 50: 80–92
Beard JS (1944) Climax vegetation in tropical America. Ecology 25: 127–158
Beard JS (1949) The natural vegetation of the Windward and Leeward Islands. Oxf For Mem 21: 1–192
Cavelier J (1986) Relaciones hidricas y de nutrientes en bosques enanos nublados. Thesis, Universidad de los Andes, ULA, Merida, Venezuela
Cavelier J, Goldstein G (1989) Mist and fog interception in elfin cloud forests in Colombia and Venezuela. J Trop Ecol 5: 309–322
Cavelier J, Mejia C (1990) Climatic factors and tree stature in the elfin cloud forest of Serrania de Macuira, Colombia. Agric For Meteorol (in press)
Chiariello D (1984) Leaf energy balance in the wet lowland tropics. In: Medina E, Mooney HA, Vazquez-Yanes C (eds) Physiological ecology of plants of the wet tropics. Junk, The Hague, pp 85–98
Fanjul L, Barradas VL (1987) Diurnal and seasonal variations in the water relations of some deciduous and evergreen trees of a deciduous dry forest of the western coast of Mexico. J Apl Ecol 24: 289–303
Farquhar GD, Schulze E-D, Küppers M (1980) Responses to humidity by stomata of Nicotiana glauca L. and Corylus avellana L. are consistent with the optimization of carbon dioxide with respect to water loss. Aust J Plant Physiol 7: 315–327
Fetcher N (1979) Water relations of five tropical tree species on Barro Colorado Island. Oecologia 40: 229–233
Gates DM (1969) The ecology of an elfin cloud forest in Puerto Rico, 4. Transpiration rates and temperatures of leaves in cool humid environments. J Arnold Arbor Harv Univ 50: 93–98
Goldstein G, Meinzer F, Monasterio M (1984) The role of capacitance in the water balance of Andean giant rosette species. Plant Cell Environ 7: 179–186
Goldstein G, Rada F, Orozco A, Montilla M, Zabala O, Cavelier J, Azocar A (1989) Mantenimiento de turgor en especies lenosas tropicales: un moqelo basado en cambios estacionales de osmolaridad y elasticidad. Monographs in systematic botany from the Missouri Botanical Garden 27: 37–48
Grace J, Okali DUU, Fasehum FE (1982) Stomatal conductance of two tropical trees during the wet season in Nigeria. J Appl Ecol 19: 659–670
Grubb PJ (1974) Factors controlling the distribution of forests-types on tropical mountains: new facts and a new perspective. In: Flenley JR (ed) Altitudinal zonation in Malesia (Miscellaneous series, 16) University of Hull Geography Department, pp 13–46
Grubb PJ (1977) Control of forest growth and distribution on wet tropical mountains: with special reference to mineral nutrition. Annu Rev Ecol Syst 8: 83–107
Grubb PJ (1986) Sclerophylls, pachyphylls and pycnophylls: the nature and significance of hard leaf surfaces: In: Juniper B, Southwood TRE (eds) Insects and plant surfaces. Arnold, London, pp 137–150
Grubb PJ, Whitmore TC (1966) A comparison of montane and lowland rain forest in Ecuador. 2. The climate and its effect on the distribution and physiognomy of the forest. J Ecol 54: 303–333
Hall AE, Schulze E-D, Lange OL (1976) Current perspectives of steadystate stomatal responses to environment. In: Lange OL, Kappen L, Schulze E-D (eds) Water and plant life, problems and modern approaches. Springer, Berlin Heidelberg New York, pp 168–188
Howard RA (1968) The ecology of an elfin forest in Puerto Rico. I. Introduction and composition studies. J Arnold Arbor Harv Univ 50: 381–418
Howard RA (1969) The ecology of an elfin forest in Puerto Rico. 8. Studies of growth and form and the leaf structure. J Arnold Arbor Harv Univ 49: 225–267
Johnston JR (1909) Flora of the islands of Margarita and Coche, Venezuela. Contrib Gray Herb Harv Univ 37: 1–149
Kapos V, Tanner EVJ (1985) Water relations of Jamaican upper montane rain forest trees. Ecology 66: 241–250
Körner C, Sheel JA, Bauer H (1979) Maximum leaf diffusive conductance in vascular plants. Photosynthetica 13: 45–82
Körner C, Allison A, Hilscher H (1983) Altitudinal variation of leaf diffusive conductance and leaf anatomy in heliophytes of montane New Guinea and their interrelations with microclimate. Flora 174: 91–135
La Bastille A, Pool DJ (1978) On the need for a system of cloud-forest parks in middle America and the Caribbean. Environ conserv 5: 183–190
Lahey JF (1958) On the origin of the dry climate in northern South America and southern Caribbean. Dep Meteorol Univ Wisconsin Sci Rep 10: 1–290
Lange OL, Losch P, Schulze E-D, Kappen L (1971) Responses of stomata to changes in humidity. Planta 100: 76–86
Lawton RO, Dryer V (1980) Vegetation of the Monteverde Cloud Forest Reserve. Brenesia 18: 101–116
Lee HS, Schmitt AK, Lüttge U (1989) The response of the C3-CAM tree, Clusia rosea, to light and water stress. J Exp Bot 40: 171–179
Macdonald WD (1964) Geology of the Serrania de Macuira, Guajira Peninsula, Colombia. Ph.D. dissertation, Princeton University
Medina E, Sobrado M, Herrera R (1978) Significance of leaf orientation for leaf temperature in an Amazonian sclerophyll vegetation. Radiat Environ Biophys 15: 131–140
Medina E, Cuevas E, Weaver, PL (1981) Composicion foliar y transpiracion de especies lenosas de Pico del Este, Sierra de Luquillo, Puerto Rico, Acta Cient Venez 32: 159–165
Meinzer F, Goldstein G (1985) Some consequences of leaf pubescence in the Andean giant rosette plant Espeletia timotensis. Ecology 66: 512–520
Meinzer F, Goldstein G, Jaimes M (1984) The effect of atmospheric humidity on stomatal control of gas exchange in two tropical coniferous species; Can J Bot 62: 591–595
Myers BJ, Robichaux RH, Unwin GL, Craig IE (1987) Leaf water relations and anatomy of a tropical rainforest tree species vary with crown position. Oecologia 74: 81–85
Oberbauer SF, Strain BR, Riechers GH (1987) Field water relations of a wet-tropical forest tree species, Pentaclethra macroloba (Mimosaceae). Oecologia 71: 369–374
Odum HT, Drewry G, Kline JR (1970) Climate at El Verde, 1963–1966. In: Odum HT, Pigeon RF (eds) A tropical rain forest. US Dept. of Commerce, Springfield, Virginia, pp B 347–418
Popp M, Kramer D, Lee H, Diaz M, Ziegler H, Lüttge U (1987) Crassulacean acid metabolism in tropical dicotyledonous trees of the genus Clusia. Trees 1: 238–247
Raunkiaer C (1934) The life forms of plants and statistical plant geography. Oxford University Press, Oxford, 632 pp
Rieger W (1976) Vegetationskundliche Untersuchungen der Guajira-Halbinsel (Nordost-Kolumbien). Giessener Geogr Schriftenr 40: 1–142
Robichaux RH (1984) Variation in the tissue water relations of two sympatric Hawaiian Dubautia species and their natural hybrid. Oecologia 65: 75–81
Robichaux RH, Rundel PW, Stemmermann L, Canfield JE, Morse SR, Friedman WE (1984) Tissue water deficits and plant growth in wet tropical environments. In: Medina E, Mooney HA, Vazquez-Yanes C (eds) Physiological ecology of plants of the wet tropics. Junk, The Hague, pp 99–112
Schulze E-D, Lange OL, Buschbom V, Kappen LA, Evenari M (1972) Stomatal responses to change in humidity in plants growing in the desert. Planta 108: 259–270
Sobrado MA (1986) Aspects of tissue water relations and seasonal changes of leaf water potential components of evergreen and deciduous species coexisting in tropical dry forest. Oecologia 68: 413–416
Sugden A (1982a) The vegetation of the Serrania de Macuira, Guajira, Colombia: a contrast of arid lowlands and an isolated cloud forest. J Arnold Arbor Harv Univ 63: 1–30
Sugden A (1982b) The ecological geography and taxonomic relationships of the flora at an isolated Colombian Cloud forest, with some implications for island biogeography. J Arnold Arbor Harv Univ 67: 31–61
Sugden A (1985) Leaf anatomy in a Venezuelan montane forests. J Linn Soc Lond Bot 90: 231–241
Sugden A (1986) The montane vegetation and flora of Margarita Island, Venezuela. J Arnold Arbor Harv Univ 67: 187–232
Tamayo F (1941) Exploraciones botanicas en la Peninsula de Paraguana, Edo. Falcon. Bol Soc Venez Cienc Nat 7: 1–90
Tanner EVT, Kapos V (1982) Leaf structure of Jamaican upper montane rain forest trees. Biotropica 14: 16–24
Tyree MT, Hammel HT (1972) The measurement of the turgor pressure and water relations of plants by the pressure-bomb technique. J Exp Bot 23: 267–283
Tyree MT, Jarvis PG (1982) Water in tissues and cells. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, new series. Physiological plant ecology II, vol 12B. Springer, Berlin Heidelberg New York, pp 35–77
Weaver PL, Byer MD, Bruck DL (1973) Transpiration rates in the Luquillo Mountains of Puerto Rico. Biotropica 5: 123–133
Webb LJ (1959) A physiognomic classification of Australian rain forests. J Ecol 47: 551–570
Whitehead D, Okali DUU, Fasehun FE (1981) Stomatal response to environmental variables in two tropical forest species during the dry season in Nigeria. J Appl Ecol 18: 571–587
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cavelier, J. Tissue water relations in elfin cloud forest tree species of Serrania de Macuira, Guajira, Colombia. Trees 4, 155–163 (1990). https://doi.org/10.1007/BF00225780
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00225780