Abstract
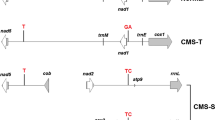
Previous results have shown that cytoplasmic male sterility (CMS) in lines from Phaseolus coccineus and Phaseolus vulgaris contain the same CMS-specific sequence, raising the question of whether this sequence rearrangement arose before divergence of the two species or afterward with subsequent transfer by introgression. Hybridization patterns of total DNA from eight P. vulgaris lines with cytoplasm from P. coccineus and three P. vulgaris lines were examined in order to analyze the mitochondrial DNA (mtDNA) diversity within each species and to determine differences between CMS lines derived from the two species. Three restriction enzymes and 17 heterologous mtDNA sequences were used. The analysis of the different hybridization patterns revealed a considerable diversity in mtDNA organization particularly within P. coccineus. We obtained distinctive hybridization patterns for the five CMS lines tested. The resulting classification showed that mitochondrial genomes from P. coccineus CMS lines group with those of fertile P. coccineus but not with CMS lines from P. vulgaris. The groupings concur with the taxonomic classification of these lines. The results support the hypothesis of a single ancient origin of the CMS determinant and exclude the transfer of cytoplasm by introgression from P. vulgaris to P. coccineus and P. coccineus ssp polyanthus.
Similar content being viewed by others
References
Bannerot H (1988) The potential of hybrid bean. In: Beebe S (ed) Current topics in breeding of the common bean. Working document no. 47. Bean program. CIAT, Cali, Colombia, pp 111–134
Bannerot H, Charbonnier L (1987) Induction de stérilités mâles cytoplasmiques par alloplasmie dans le genre Phaseolus: étude génétique et moléculaire. In: Bervillé A (ed) Variabilité génétique cytoplasmique et stérilité mâle cytoplasmique. INRA Editions, pp 181–198
Bassett MJ, Shuh DM (1982) Cytoplasmic male sterility in common bean. J Am Soc Hort Sci 107(5):791–793
Bland MM, Levings, III CS, Matzinger DF (1987) The ATPase subunit 6 gene of tobacco mitochondria contains an unusual sequence. Curr Genet 12:475–481
Bonhomme S, Budar F, Férault M, Pelletier G (1991) A 2.5-kb NcoI fragment of Ogura radish mitochondrial DNA is correlated with cytoplasmic male-sterility in Brassica cybrids. Curr Genet 19:121–127
Chase C, Ortega V (1992) Organisation of atpA and 3′ flanking sequences associated with cytoplasmic male sterility in Phaseolus vulgaris L. Curr Genet 22:147–153
Crouzillat D, Leroy P, Perrault A, Ledoigt G (1987) Molecular analysis of the mitochondrial genome of Helianthus annuus in relation to cytoplasmic male sterility and phylogeny. Theor Appl Genet 74:773–780
Dawson A, Jones VP, Leaver CJ (1984) The apocytochrome b gene in maize mitochondria does not contain introns and is preceded by a potential ribosome binding site. EMBO J 3:2107–2113
Dellaporta SL, Wood J, Hicks JP (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Gualberto JM, Wintz H, Weil JH, Grienenberger JM (1988) The genes coding for subunit 3 of NADH dehydrogenase and for ribosomal protein S12 are present in the wheat and maize mitochondrial genomes and are co-transcribed. Mol Gen Genet 215:118–127
Gualberto JM, Weil J-H, Grienenberger J-M (1990) Editing of the wheat coxIII transcript: evidence for twelve C to U and one U to C conversion and for the sequence similarities around editing sites. Nucleic Acids Res 18:3771–3776
Hervieu F, Charbonnier L, Bannerot H, Pelletier G (1993) The cytoplasmic male sterility (CMS) determinant of common bean is widespread in Phaseolus vulgaris L. and Phaseolus coccineus L. Curr Genet 24:149–155
Johns C, Meiquing L, Lyznik A, Mackenzie S (1992) A mitochondrial DNA sequence is associated with abnormal pollen development in cytoplasmic male-sterile bean plants. Plant Cell 4:435–449
Khairallah MM, Adams MW, Sears BB (1990) Mitochondrial DNA polymorphism of Malawian beaan lines: further evidence for two major gene pools. Theor Appl Genet 80:753–760
Khairallah MM, Adams MW, Sears BB (1991) Mitochondrial genome size variation and restriction fragment length polymorphism in three Phaseolus species. Theor Appl Genet 82:321–328
Lamattina L, Weil JH, Grienenberger JM (1989) RNA editing at a splicing site of an NADH dehydrogenase subunit IV gene transcript in wheat mitochondria. FEBS Lett 258:79–83
Lejeune B, Delorme S. Delcher E, Quetier F (1987) Recombination in wheat mitochondrial DNA: occurence of nine different genomic contexts for the 18s-5s genes. Plant Physiol Biochem 25:227–233
Mackenzie SA (1991) Identification of a sterility-inducing cytoplasm in a fertile accession line of Phaseolus vulgaris L. Genetics 127:411–416
Mackenzie S, Chase C (1990) Fertility restoration is associated with loss of a portion of the mitochondrial genome in cytoplasmic male-sterile common bean. Plant Cell 4:905–912
Mackenzie SA, Pring DR, Bassett MJ, Chase CD (1988) Mitochondrial DNA rearrangement associated with fertility restoration and reversion to fertility in cytoplasmic male sterility in Phaseolus vulgaris L. Proc Natl Acad Sci USA 85:2714–2717
Maréchal R, Mascherpa J, Stainier F (1978) Etude taxonomique d'un groupe complexe d'espèces des genres Phaseolus et Vigna (Papilionaceae) sur la base de données morphologiques et polliniques, traitées par l'analyse informatique. Boissiera 28:1–273
Miller JC (1990) Concerning Restsite package v1.1. University of Wisconsin
Miranda-Colin S (1979) Evolucion de Phaseolus vulgaris y P. coccineus. In Colegio de Postgraduados (eds) Contribuciones al conocimiento del frijol (Phaseolus) en Mexico. Chapingo, Mexico pp 83–99
Nei M, Li W-H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Ogura H (1968) Studies on the new male sterility in japanese radish, with special reference to the utilisation of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima University 6:39–78
Palmer JD (1992) Mitochondrial DNA in plant systematics: applications and limitations. In: Soltis PS, Soltis DE, Doyle JJ (eds) Molecular systematics in plants. Chapman and Hall, New York, pp 36–49
Palmer JD, Herbon LA (1988) Plant mitochondrial DNA evolves rapidly in structure but slowly in sequence. J Mol Evol 28:87–97
Pereira de Souza A, Jubier M-F, Delcher E, Lancelin D, Lejeune B (1991) A trans-splicing model for the expression of the tripartite nad5 gene in wheat and maize mitochondria. Plant Cell 3:1363–1378
Pring DR Levings, III CS (1978) Heterogeneity of maize cytoplasmic genomes among male-sterile cytoplasms. Genetics 89:121–136
Ricard B, Lejeune B, Araya A (1986) Studies on wheat mitochondrial DNA organization. Comparison of the mitochondrial DNA from normal and cytoplasmic male-sterile varieties of wheat. Plant Sci 43:141–149
Rieseberg LH, Beckstrom-Sternberg SM, Liston A, Arias D (1991) Phylogeny and systematic inferences from chloroplast DNA and isozyme variation in Helianthus sect. Helianthus (Asteraceae). Systematic Bot 16:50–76
Rieseberg LH, Soltis DE (1991) Phylogenetic consequences of cytoplasmic gene flow in plants. Evol Trends Plants 5:65–84
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Schmit V, Debouck DG (1991) Observations on the origin of Phaseolus polyanthus Greenman. Econ Bot 45 (3):345–364
Singh S, White J, Gutierrez (1980) Male sterility in dry beans. Annu Rep Bean Improv Coop 23:55–57
Terachi T, Tsunewaki K (1992) The molecular basis of genetic diversity among cytoplasms of Triticum and Aegilops. VIII. Mitochondrial RFLP analyses using cloned genes as probes. Mol Biol Evol 9:917–931
Timothy DA, Levings, III CS, Pring DR, Conde MF, Kermicle JC (1979) Organelle DNA variation and systematic relationships in the genus Zea: Teosinte. Proc Natl Acad Sci USA 76:4220–4224
Author information
Authors and Affiliations
Additional information
Communicated by J. S. Beckmann
Rights and permissions
About this article
Cite this article
Hervieu, F., Bannerot, H. & Pelletier, G. A unique cytoplasmic male sterility (CMS) determinant is present in three Phaseolus species characterized by different mitochondrial genomes. Theoret. Appl. Genetics 88, 314–320 (1994). https://doi.org/10.1007/BF00223638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00223638