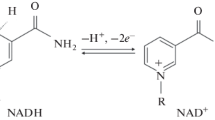
Summary
Oxidation of NADH by rat erythrocyte plasma membrane was stimulated by about 50-fold on addition of decavanadate, but not other forms of vanadate like orthovanadate, metavanadate aad vanadyl sulphate. The vanadate-stimulated activity was observed only in phosphate buffer while other buffers like Tris, acetate, borate and Hepes were ineffective. Oxygen was consumed during the oxidation of NADH and the products were found to be NAD+ and hydrogen peroxide. The reaction had a stoichiometry of one mole of oxygen consumption and one mole of H2O2 production for every mole of NADH that was oxidized.
Superoxide dismutase and manganous inhibited the activity indicating the involvement of superoxide anions. Electron spin resonance in the presence of a spin trap, 5, 5′-dimethyl pyrroline N-oxide, indicated the presence of superoxide radicals. Electron spin resonance studies also showed the appearance of VIV species by reduction of VV of decavanadate indicating thereby participation of vanadate in the redox reaction. Under the conditions of the assay, vanadate did not stimulate lipid peroxidation in erythrocyte membranes. Extracts from lipid-free preparations of the erythrocyte membrane showed full activity. This ruled out the possibility of oxygen uptake through lipid peroxidation. The vanadate-stimulated NADH oxidation activity could be partially solubilized by treating erythrocyte membranes either with Triton X-100 or sodium cholate. Partially purified enzyme obtained by extraction with cholate and fractionation by ammonium sulphate and DEAE-Sephadex was found to be unstable.
Similar content being viewed by others
References
Erdmann E, Krawietz W, Phillip G, Hackbarth I, Schmitz W, Scholz H, Crane FL: Nature (London) 282:335–336, 1979.
Crane FL, MacKellar WC, Moore DJ, Ramasarma T, Goldenberg H, Grebing C, Low H: Biochem Biophys Res Commun 93:746–754, 1980.
Menon AS, Rau M, Ramasarma T, Crane FL: FEBS Lett 114:139–141, 1980.
Ramasarma T, MacKellar WC, Crane FL: Biochim Biophys Acta 646:88–98, 1981.
Vyskocil F, Teisinger J, Dlouha H: Nature (London) 286:516–517, 1980.
Erdmann E, Krawietz W: Nature (London) 294:288, 1981.
Vyskocil F, Dlouha H, Teisinger J: Nature (London) 294:288, 1981.
Ramasarma T, MacKellar W, Crane FL: Ind J Biochem Biophys 17:163–167, 1980.
Ramasarma T, Swaroop A, MacKellar W, Crane FL: J Bioenerg Biomemb 13:241–253, 1981.
Bernstein F, Bernheim MLC: J Biol Chem 127:353–360, 1939.
Wills ED: Biochem J 113:315–324, 1969.
Dodge JT, Mitchell C, Manahau DJ: Arch Biochem Biophys 100:119–130, 1963.
Swanson PD, Bradford HP, McIlwain H: Biochem J 92:235–247, 1964.
Wilbur KM, Bernheim F, Shapiro OW: Arch Biochem 24:305–313, 1949.
Loschen G, Flohe L, Chance B: FEBS Lett. 18:261–264, 1971.
Thurman RG, Ley HG, Scholz R: Eur J Biochem 25:420–430, 1972.
Pope MT, Dale BW: Quart Rev 22:527–548, 1968.
Fridovich I: J Biol Chem 245:4053–4057, 1970.
Finkelstein E, Rosen GM, Rauckman EJ: Mol Pharmacol 16:676–685, 1979.
Grover TA, Piette LH: Arch Biochem Biophys 212:105–114, 1981.
Beinert H, Palmer G: Adv Enzymol 27:185–198, 1965.
Halliwel B: Planta 140:81–88, 1978.
Reinards R, Kubicki J, Ohlenbusch HD: Eur J Biochem 120:329–337, 1981.
Ramasarma T: Biochim Biophys Acta 694:69–93, 1982.
Ramasarma T, Crane FL: In: Horecker BL, Stadtman ER (eds), Current Topics in Cellular Regulation. 1981, Vol 20, pp 247–301.
Cantley LC Jr, Josephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G: J Biol Chem 252:7421–7423, 1977.
Schwabe U, Puchstein C, Hannemann H, Söchtig E: Nature (London) 277:143–145, 1979.
Choate G, Mansour TE: J Biol Chem 254:11457–11462, 1979.
Low H, Crane FL: FEBS Lett 68:157–159, 1976.
Vijaya S, Meera Rau, Manjunath R, Satish Menon A, Rama-Krishna Kurup CK, Ramasarma T: Curr Sci 49:620–621, 1980.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vijaya, S., Crane, F.L. & Ramasarma, T. A vanadate-stimulated NADH oxidase in erythrocyte membrane generates hydrogen peroxide. Mol Cell Biochem 62, 175–185 (1984). https://doi.org/10.1007/BF00223308
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00223308