Abstract
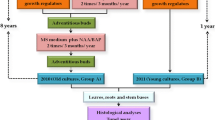
The behaviour of Brassica campestris (2n=20, AA), B. oleracea (2n=18, CC), and B. napus (2n=38, AACC) were studied during a tissue-culturing process. Hypocotyl-protoplasts were cultivated into calli from which new plants were regenerated. The regenerated plants were compared, and mitotic root-tip cells were C-banded and karyotyped. A majority of the plants were tetraploid. The meioses were studied in the PMCs. A number of abberations were observed, mainly due to faulty spindle function. There was a difference between the three species in that B. campestris performed the most poorly with many fewer regenerated plants. These plants were more morphologically disturbed and had more problems during pollen production than B. oleracea and B. napus plants.
Similar content being viewed by others
Reference
Ashmore SE, Gould AR (1981) Karyotype evolution in a tumour derived plant tissue culture analysed by Giemsa C-banding. Protoplasma 106:297–308
Bai D, Knott DR (1993) The effects of level of 2,4-D and time in culture on regeneration rate and chromosome numbers of regenerates from calli of the hybrid Triticum aestivum cv. Chinese Spring ph1b x Thinopyrum ponticum (2n=10 x=70). Genome 36:166–172
Chen BY, Heneen WK, Simonsen V (1989) Comparative and genetic studies of isozymes in resynthesized and cultivated Brassica napus L., B. campestris l. and B. alboglabra Bailey. Theor Appl Genet 77:673–679
Chen BY, Heneen WK, Simonsen V (1990) Genetics of isozyme loci in Brassica campestris L. and in the progeny of a trigenomic hybrid between B. napus L. and B. campestris L. Genome 33:433–440
D'Amato F (1964) Endopolyploidy as a factor in plant tissue development. Caryologia 17:41–52
D'Amato F (1985) Cytogenetics of plant cell and tissue cultures and their regenerates. CRC Crit Rev Plant Sci 3:73–112
Fahlesson J, Dixelius J, Sundberg E, Glimelius K(1988) Correlation between flow cytometric determination of nuclear DNA content and chromosome number in somatic hybrids within Brassicaceae by flow sorting. Plant Sci 45:133–141
Fransz PF, Leunissen EHM, Van Eeuwijk FA, Keizer PLC, Colijn-Hooymans TM (1994) Improvement of protoplast regeneration from a recalcitrant inbred line of Brassica oleracea:a morphogenetic analysis. Plant Sci 98:87–95
Geier T (1991) Chromosome variability in callus produced plants. In: Harding J, Singh F, Mol JNM (eds) Genetics and breeding of ornamental species, 11. Kluwer Academic Pub, Dordrecht, The Netherlands, pp 79–106
Glimelius K (1984) High growth rate and regeneration capacity of hypocotyl protoplasts in some Brassicaceae. Physiol Plant 61:38–44
Jourdan PS, Earle ED (1989) Genotypic variability in the frequency of plant regeneration from leaf protoplasts of four Brassica ssp. and Raphanus sativus. J Am Soc Hortic Sci 114:343–349
Kao KN, Michalyk MR (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110
Linde-Laursen I, von Bothmer R (1993) Abberant meiotic divisions in a Hordeum lechleri x H. Vulgare hybrid. Hereditas 118:145–153
McGrath JM, Quiros CF, Harada JJ, Landry BS (1990) Identification of Brassica oleracea monosomic alien chromosome addition lines with molecular markers reveals extensive gene duplication. Mol Gen Genet 223:198–204
Newell CA, Rhoads ML, Bidney DL (1984) Cytogenetic analysis of plants regenerated from tissue explants and mesophyll protoplasts of winter rape, Brassica napus L. Can J Genet Cytol 26:752–761
Olin-Fatih M (1994) A new method for differential staining of Brassica campestris L., B. oleracea L. and B. napus L. Hereditas 120:253–259
Olin-Fatih M, Heneen WK (1992) C-banded karyotypes of Brassica campestris, B. oleracea, and B. napus. Genome 35:583–589
Papes D, Garaj-Vrhovac V, Jelaska S, Kolevska-Pletikapic B (1983) Chromosome behavior in cultured cell populations of higher plants. In: Brandham PE, Bennett MD (eds) Kew chromosome conference II. George Allen and Unwin, London, pp 155–163
Quiros CF, Ochoa O, Kianian SF, Douches D (1987) Analysis of the Brassica oleracea genome by the generation of B. campestrisoleracea chromosome addition lines: characterization by isozyme and rDNA genes. Theor Appl Genet 74:758–766
Simmonds DH (1992) Plant cell wall removal:cause for microtubule instability and division abnormalities in protoplast cultures? Physiol Plant 85:387–390
Slocum MK, Figdore SS, Kennard WC, Suzuki JY, Osborn TC (1990) Linkage arrangement of restriction fragment length polymorphism loci in Brassica oleracea. Theor Appl Genet 80:57–64
Song KM, Suzuki JY, Slocum MK, Williams PH, Osborn TC (1991) A linkage map of Brassica rapa (syn. campestris) based on restriction fragment length polymorphism loci. Theor Appl Genet 82:296–304
Sundberg E (1991) Somatic hybrids and cybrids within Brassicaceae. PhD thesis., Uppsala
U N (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot 7:389–452
Zhao K-N, Whitecross MI, Bittisnich DJ (1994) Studies on plant regeneration from cotyledonary protoplasts in Brassica campestris. Plant Cell Rep 13:164–170
Author information
Authors and Affiliations
Additional information
Communicated by P. M. A. Tigerstedt
Rights and permissions
About this article
Cite this article
Olin-Fatih, M. The morphology, cytology, and C-banded karyotypes of Brassica campestris, B. oleracea, and B. napus plants regenerated from protoplasts. Theoret. Appl. Genetics 93, 414–420 (1996). https://doi.org/10.1007/BF00223184
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00223184