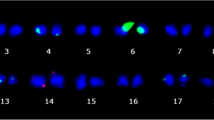
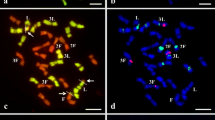
Abstract
Plant regeneration from cotyledons of seeds of a single progeny of a pure line of Helianthus annuus was studied in respect of the nuclear DNA contents of control and regenerated plants. Control plants were divided into two groups: those developed from seeds at the periphery of the inflorescence (showing a high basic 4C DNA content) and those from seeds developed in the middle of the inflorescence (showing a low basic 4C DNA content). It was observed that plants from peripheral seeds have a higher morphogenetic potential than those from central seeds. Cytophotometric analyses indicated that plants regenerated from cotyledons of both peripheral and central seeds show the same basic 4C DNA amount, which is higher that that observed in vivo in peripheral seeds. Molecular analysis by slot blotting and hybridization with different DNA families showed that the difference in nuclear DNA content between plants from peripheral and central seeds in vivo are mainly related to differences in the frequency of highly repeated, “slow” medium repeated (MR2), and ribosomal DNA families; by contrast, the increase in DNA amount in regenerated plants is mainly due to “fast” medium repeated sequences (MR1). Moreover, the frequency of kinetically isolated “unique” sequences was higher in peripheral seeds than in central ones and still higher in regenerated plants. Optical-density measurements of interphase nuclei showed an increase of heterochromatin in regenerated plants, suggesting that, whatever DNA is amplified in these plants, it remains condensed and probably inactive.
Similar content being viewed by others
References
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Bebeli PJ, Kaltsikes PJ, Karp A (1993) Field evaluation of somaclonal variation in rye lines differing in telomeric heterochromatin. J Genet Breed 47:15–22
Bennett MD (1972) Nuclear DNA content and minimum generation time in herbaceous plants. Phil Trans Roy Soc Lond, B 181:109–135
Bennett MD (1987) Variation in genomic form in plants and its ecological implications. New Phytol 106:177–200
Biradar DP, Bullock DG, Lane Rayburn A (1994) Nuclear DNA amount, growth, and yield parameters in maize. Theor Appl Genet 88:557–560
Brettell RIS, Pallotta MA, Gustafson JP, Appels R (1986) Variation at the Nor loci in triticale derived from tissue culture. Theor Appl Genet 71:637–643
Britten RJ, Graham DE, Neufeld BR (1974) Analysis of repeating DNA sequences by reassociation. Methods Enzymol 29:363–405
Brown PTH, Lörz H (1986) Molecular changes and possible origins of somaclonal variation. In: Semal J (ed) Somaclonal variations and crop improvement. Martinus Nijhoff, Dordrecht, pp 148–159
Brown PTH, Göbel E, Lörz H (1991) RFLP analysis of Zea mays callus cultures and their regenerated plants. Theor Appl Genet 81:227–232
Cavallini A, Natali L (1989) Cytological analysis of in vitro somatic embryogenesis in Brimeura amethystina Salisb. (Liliaceae). Plant Sci 62:255–261
Cavallini A, Natali L (1991) Intraspecific variation of nuclear DNA content in plant species. Caryologia 44:93–107
Cavallini A, Zolfino C, Cionini G, Cremonini R, Natali L, Sassoli O, Cionini PG (1986) Nuclear DNA changes within Helianthus annuus L.: cytophiotometric, karyological and biochemical analysis. Theor Appl Genet 73:20–26
Cavallini A, Zolfino C, Natali L, Cionini G, Cionini PG (1989) Nuclear DNA changes within Helianthus annuus L.: origin and control mechanism. Theor Appl Genet 77:12–16
Cavallini A, Natali L, Cionini G, Gennai D (1993a) Nuclear DNA variability within Pisum sativum (Leguminosae): nucleotypic effects on plant growth. Heredity 70:561–565
Cavallini A, Natali L, Cionini G, Sassoli O, Castorena-Sanchez I (1993b) In vitro culture of Aloe barbadensis Mill.: quantitative DNA variations in regenerated plants. Plant Sci 91:223–229
Cecchini E, Natali L, Cavallini A, Durante M (1992) DNA variations in regenerated plants of pea (Pisum sativum L.). Theor Appl Genet 84:874–879
Cullis CA (1990) DNA rearrangements in response to environmental stress. Adv Genet 28:73–97
Cullis CA, Cleary W (1986) DNA variation in flax tissue culture. Can J Genet Cytol 28:247–251
Dennis GS, Gerlach WI, Pryor AJ, Bennetzen JL, Inglis A, Llewellyn D, Sachs MM, Ferl RJ, Peacock WJ (1984) Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res 12:3893–4000
DePaepe R, Prat D, Huguet T (1982) Heritable nuclear-DNA changes in doubled haploid plants obtained by pollen culture of Nicotiana sylvestris. Plant Sci Lett 28:11–28
Deumling B, Clermont L (1989) Changes in DNA content and chromosomal size during cell culture and plant regeneration of Scilla siberica: selective chromatin diminution in response to environmental conditions. Chromosoma 97:439–448
Dhillon SS, Wernsman EA, Miksche JP (1983) Evaluation of nuclear DNA content and heterochromatin changes in anther-derived dihaploids of tobacco (Nicotiana tabacum) cv Coker 139. Can J Genet Cytol 25:169–173
Doyle JJ, Doyle JL (1989) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Evans GM, Durrant A, Rees H (1966) Associated nuclear changes in the induction of flax genotrophs. Nature 212:697–699
Karp A (1991) On the current understanding of somaclonal variation. Oxford Surv Plant Mol Cell Biol 7:1–58
Kidwell KK, Osborn TC (1993) Variation among alfalfa somaclones in copy number of repeated DNA sequences. Genome 36:906–912
Kikuchi S, Takaiwa F, Oono K (1987) Variable copy number DNA sequences in rice. Mol Gen Genet 210:373–380
Larkin PJ, Scowcroft WR (1981) Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Landsmann J, Uhrig H (1985) Somaclonal variation in Solanum tuberosum detected at the molecular level. Theor Appl Genet 71:500–505
Maggini F, Tucci GF, Demartis A, Gelati MT, Avanzi S (1992) Ribosomal RNA genes in Phaseolus coccineus. Plant Mol Biol 18:1073–1082
Michaelson MJ, Price HJ, Spencer Johnston J, Ellison JR (1991) Variation of nuclear DNA content in Helianthus annuus (Asteraceae). Am J Bot 78:1238–1243
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagl W (1990) Gene amplification and related events. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 11. Somaclonal variation in crop improvement I. Springer, Berlin, pp 153–201
Nagl W, Capesius I (1976) Molecular and cytological characteristics of nuclear DNA and chromatin for angiosperm systematics: basic data for Helianthus annuus (Asteraceae). Plant Syst Evol 126:221–237
Natali L, Cavallini A, Cremonini R, Bassi P, Cionini PG (1986) Amplification of nuclear DNA sequences during induced plant cell de-differentiation. Cell Diff 18:157–161
Natali L, Cavallini A, Cionini G, Sassoli O, Cionini PG, Durante M (1993) Nuclear DNA changes within Helianthus annuus L.: change within single progenies and their relationships to plant development. Theor Appl Genet 85:506–512
Olszewska MJ, Osiecka R (1983) The relationship between 2C DNA content, life-cycle type, systematic position, and the dynamics of DNA endoreduplication in parenchyma nuclei during growth and differentiation of roots in some dicotyledonous herbaceous species. Biochem Physiol Pflanz 178:581–589
Peschke VM, Phillips RL (1992) Genetic implications of somaclonal variation in plants. Adv Genet 30:41–75
Price HJ (1988) Nuclear DNA content variation within angiosperm species. Evol Trends Plants 2:53–60
Pugliesi C, Cecconi F, Mandolfo A, Baroncelli S (1991) Plant regeneration and genetic variability from tissue cultures of sunflower(Helianthus annuus L.). Plant Breed 106:114–121
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Scheffe' H (1959) The analysis of variance. J Wiley and Sons Inc, London
Schneeberger RG, Cullis CA (1991) Specific DNA alterations associated with the environmental induction of heritable changes in flax. Genetics 128:619–630
Smyth DR (1991) Dispersed repeats in plant genomes. Chromosoma 100:355–359
Walbot V, Cullis CA (1985) Rapid genome changes in higher plants. Annu Rev Plant Physiol 36:367–396
Author information
Authors and Affiliations
Additional information
Communicated by F. Mechelke
Research supported by National Research Council of Italy, Special Project RAISA, Sub-project No. 2, Paper No. 2069
Rights and permissions
About this article
Cite this article
Natali, L., Giordani, T., Cionini, G. et al. Heterochromatin and repetitive DNA frequency variation in regenerated plants of Helianthus annuus L.. Theoret. Appl. Genetics 91, 395–400 (1995). https://doi.org/10.1007/BF00222965
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222965