Summary
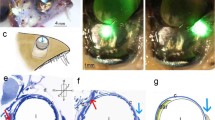
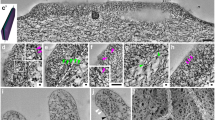
The so-called ‘basement membrane’ of arthropod compound eyes is known to be of heterogeneous origin (Odselius and Eloffson 1981). A major contribution in Diptera with open rhabdoms is provided by a pigmented component which lies at the basal end of the extracellular space of each ommatidium and fills it, the glial plug. Ancillary components consist of the expanded tips of cone cell processes. Each glial plug exhibits two distinct regions: ramifying processes extend into the extracellular space and contain numerous pigment granules, while proximally the cytoplasm is devoid of granules but packed with bundles of cross-linked microfilaments that bind the fluorescent F-actin probe NBD-phallacidin strongly and antibodies to scallop actin weakly. Cone cell expansions also contain microfilaments and exhibit the same binding properties. The proximal faces of the cells of the glial plugs and of the cone cell expansions are covered with a coarsely fibrillar extracellular matrix. Some actin bundles appear to be attached to the plasma membranes at their ends, although the reality of this arrangement is still in question. Cellular components of the basement membrane are bonded together by their extracellular matrices, so that collectively they provide a reinforced network that retains the retina. Bundles of axons from the photoreceptors and tracheae that supply the retina with tracheoles pass through the spaces in this network.
Similar content being viewed by others
References
Barak LS, Yocum RR (1981) NBD-phallacidin: synthesis of a fluorescent actin probe. Anal Biochem 110:31–38
Blest AD, Stowe S, Eddey W (1982a) A labile, Ca2+-dependent cytoskeleton in rhabdomeral microvilli of blowflies. Cell Tissue Res. 223:553–573
Blest AD, Stowe S, Eddey W, Williams DS (1982b) The local deletion of a microvillar cytoskeleton from photoreceptors of tipulid flies during membrane turnover. Proc R Soc Lond B 215:469–479
Blest AD, Eddey W (1983) The extra-rhabdomeral cytoskeleton in photoreceptors of Diptera: II. Sub-membrane cytoskeletons (in preparation)
Boschek BC (1971) On the fine structure of the peripheral retina and lamina ganglionaris of the fly Musca domestica. Z Zellforsch 118:369–409
De Couet HG, Gröschel-Stewart U (1983) Studies on the antigenic sites of actin: a comparative study of immunogenic cross-reactivity of invertebrate actins. J Muscle Res Cell Motil (in the press)
De Couet HG, Mazander KD, Gröschel-Stewart U (1980) A study of invertebrate actins by isoelectric focusing and immunodiffusion. Experientia 36:404–405
De Couet HG, Stowe S, Blest AD (1983) Membrane-associated actin in the rhabdomeral microvilli of crayfish photoreceptors. J Cell Biol (submitted)
Drenckhahn D, Kellner J, Mannherz HG, Gröschel-Stewart U, Kendrick-Jones J, Scholey J (1982) Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature (Lond) 300:531–532
Falkner F-G, Saumweber H, Biessman H (1981) Two Drosophila melanogaster proteins related to intermediate filament proteins of vertebrate cells. J Cell Biol 91:175–183
Kamakura K, Ihara Y, Sugita H, Toyokura Y (1981) Inhibition by E-64-c of neurofilament degeneration induced by calcium ions. Biomed Res 2:327–329
Melamed J, Trujillo-Cenoz O (1968) The fine structure of the central cells in the ommatidia of dipterans. J Ultrastruct Res 21:313–334
Nothnagel EAL, Barak LS, Sanger JW, Webb WW (1981) Fluorescence studies on modes of cytochalasin B and phallotoxin action on cytoplasmic streaming in Chara. J Cell Biol 88:364–372
Odselius R, Eloffson R (1981) The basement membrane of the insect and crustacean compound eye: definition, fine structure and comparative morphology. Cell Tissue Res 216:205–214
Pruss RM, Mirsky R, Raff MC, Thorpe AJ, Dowding S, Anderton BH (1981) All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell 27:419–428
Shaw SR (1978) The extracellular space and blood-eye barrier in an insect retina: an ultrastructural study. Cell Tissue Res 188:35–61
Singer II (1979) The fibronexus: a transmembrane association of fibronectin-containing fibers, and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16:675–685
Singer II, Paradiso PR (1981) A transmembrane relationship between fibronectin and vinculin (130 KD protein): serum modifications in normal and transformed hamster fibroblasts. Cell 16:675–685
Tamai M, Hanada K, Adachi T, Oguma K, Kashiwagi K, Omura S, Ohzeki M (1981) Papain inhibitions by optically active E-64 analogues. J Biochem 90:255–257
Tilney LG, Derosier DG, Mulroy MJ (1980) The organisation of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol 86:244–259
Author information
Authors and Affiliations
Additional information
The authors thank Tom Van Gieren and Peter Dunlop for supplying Lucilia from CSIRO cultures, and Jonathon Howard for the nss mutant; Wendy Eddey and Claudia Sigmund for cutting thin sections, and George Weston and the Staff of the ANU Transmission Electron Microscopy Unit for support. Ep-475 was a generous gift from the Taisho Pharmaceutical Company Ltd., Tokyo, and Dr. K. Hanada. Professor Martin Raff and Dr. H. Saumweber provided monoclonal antibodies to vertebrate and Drosophila intermediate filaments, respectively. Antibodies to scallop actin were raised by H.G. de C. at the Technische Hochschule, Darmstadt in the laboratory of Professor Ute Gröschel-Stewart. Dr. Sally Stowe provided many helpful discussions
Rights and permissions
About this article
Cite this article
Blest, A.D., De Couet, H.G. Actin in cellular components of the basement membrane of the compound eye of a blowfly. Cell Tissue Res. 231, 325–336 (1983). https://doi.org/10.1007/BF00222184
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222184