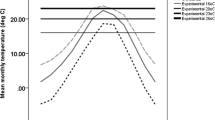
Summary
Body size in Drosophila is known to be closely related to a number of traits with important life history consequences, such as fecundity, dispersal ability and mating success. We examine the quantitative genetic basis of body size in three populations of the cactophilic species Drosophila buzzatii, which inhabit climatically different areas of Australia. Flies were reared individually to eliminate any common environmental component in a full-sib design with families split between two temperatures (18° and 25 °C). The means of several size measures differ significantly among populations while the genetic correlations among these traits generally do not differ, either among populations from different natural environments or between the different laboratory temperatures. This stability of correlation structure is necessary if laboratory estimates of genetic correlations are to have any connection with the expression of genetic variation in the field. The amount of variance due to genotype-by-environment interactions (family x temperature of development) varied among populations, apparently in parallel with the magnitudes of seasonal and diurnal variation in temperature experienced by the different populations. A coastal population, inhabiting a relatively thermally benign environment, showed no interaction, while two inland populations, inhabiting thermally more extreme areas, showed interaction. This interaction term is a measure of the amount of genetic variation in the degree of phenotypic plasticity of body size in response to temperature of development. Thus the inland flies vary in their ability to attain a given body size at a particular temperature while the coastal flies do not. This phenotypic plasticity is shown to be due primarily to differences among genotypes in the amount of response to the change in temperature. A possible selective basis for the maintenance of genetic variation for the levels of phenotypic plasticity is proposed.
Similar content being viewed by others
References
Atchley WR, Gaskins CT, Anderson D (1976) Statistical properties of ratios. I. Empirical results. Syst Zool 25:137–148
Atkinson WD (1979) A comparison of the reproductive strategies of domestic species of Drosophila. J Anim Ecol 48:53–64
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Adv Genet 13:115–155
Barker JSF, Mulley JC (1976) Isozyme variation in natural populations of Drosophila buzzatii. Evolution 30:213–233
Barker JSF, Sene FdeM, East PD, Pereira MAQR (1985) Allozyme and chromosomal polymorphism of Drosophila buzzatii in Brazil and Argentina. Genetica 67:161–170
Bowman JC (1972) Genotype x environment interactions. Ann Génét Sél anim 4:117–123
Cavicchi S, Guerra D, Giorgi G, Pezzoli C (1985) Temperaturerelated divergence in experimental populations of Drosophila melanogaster. I. Genetic and developmental basis of wing size and shape variation. Genetics 109:665–689
Clark AG (1987) Senescence and the genetic-correlation hangup. Am Nat 129:932–940
Coyne JA, Beecham E (1987) Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics 117:727–737
de Jong G (1990a) Quantitative genetics of reaction norms. J Evol Biol 3:447–468
de Jong G (1990b) Genotype-by-environment interaction and the genetic covariance between environments: multilocus genetics. Genetica 81:171–177
Dickerson GE (1962) Implications of genetic-environmental interaction in animal breeding. Anim Prod 4:47–63
Falconer DS (1989) Introduction to quantitative genetics, 3rd edn. Longman, Harlow, England
Falconer DS, King JWB (1953) A study of selection limits in the mouse. J Genet 51:561–581
Gabriel W, Lynch M (1992) The selective advantage of reaction norms for environmental tolerance. J Evol Biol 5:41–59
Garcia-Bellido A, Ripoll P, Morata G (1973) Developmental compartmentalisation of the wing disk of Drosophila. Nature 245:251–253
Gomulkiewicz R, Kirkpatrick M (1992) Quantitative genetics and the evolution of reaction norms. Evolution 46:390–411
Gupta AP, Lewontin RC (1982) A study of reaction norms in natural populations of Drosophila pseudoobscura. Evolution 36:934–948
Haldane JBS (1946) The interaction of nature and nurture. Ann Eugen 13:197–205
Harvey WR (1982) Mixed model capabilities of LSML76. J Anim Sci 54:1279–1285
Harvey WR (1988) User's Guide for LSMLMW. PC-1 Version. Copyright WR Harvey
Hedrick PW (1976) Genetic variation in a heterogeneous environment. II. Temporal heterogeneity and directional selection. Genetics 84:145–157
Hedrick PW (1986) Genetic polymorphism in heterogeneous environments: a decade later. Annu Rev Ecol Syst 17:535–566
Levins R (1969) Thermal acclimation and heat resistance in Drosophila species. Am Nat 103:483–499
Lynch M, Gabriel W (1987) Environmental tolerance. Am Nat 129:283–303
McFarquhar AM, Robertson FW (1963) The lack of evidence for co-adaptation in crosses between geographical races of Drosophila subobscura Coll. Genet Res 4:104–131
Misra RK, Reeve ECR (1964) Clines in body dimension in populations of Drosophila subobscura. Genet Res 5:240–256
Orzack SH (1985) Population dynamics in variable environments V. The genetics of homeostasis revisited. Am Nat 125:550–572
Pani SN, Lasley JF (1972) Genotype x environment interactions in animals. Res Bull 992. University of Missouri-Columbia, Missouri, USA
Partridge L, Ewing A, Chandler A (1987a) Male size and mating success in Drosophila melanogaster: the roles of male and female behaviour. Anim Behav 35:555–562
Partridge L, Hoffmann A, Jones JS (1987b) Male size and mating success in Drosophila melanogaster and D. pseudoobscura under field conditions. Anim Behav 35:468–476
Pfriem P (1983) Latitudinal variation in wing size in Drosophila subobscura and its dependence on polygenes in chromosome O. Genetica 61:221–232
Prevosti A (1955) Geographical variability in quantitative traits in populations of Drosophila subobscura. Cold Spring Harbor Symp Quant Biol 20:294–299
Prout T, Barker JSF (1989) Ecological aspects of the heritability of body size in Drosophila buzzatii. Genetics 123:803–813
Reeve ECR, Robertson FW (1953) Analysis of environmental variability in quantitative inheritance. Nature 171:874–875
Robertson A (1959) The sampling variance of the genetic correlation coefficient. Biometrics 15:469–485
Robertson FW (1957) Studies in quantitative inheritance XI. Genetic and environmental correlation between body size and egg production in Drosophila melanogaster. J Genet 55:428–443
Robertson FW (1987) Variation of body size within and between wild populations of Drosophila buzzatii. Genetica 72:111–125
Robertson FW, Reeve E (1952) Studies in quantitative inheritance I. The effects of selection of wing and thorax length in Drosophila melanogaster. J Genet 50:414–448
Robertson FW, Reeve E (1955) Studies in quantitative inheritance. VIII. Further analyses of heterosis in crosses between inbred lines of Drosophila melanogaster. Z indukt Abstamm Vererblehre 86:439–458
Roff D (1977) Dispersal in dipterans: Its costs and consequences. J Anim Ecol 46:443–456
Roff DA, Mousseau TA (1987) Quantitative genetics and fitness: lessons from Drosophila. Heredity 58:103–118
Ruiz A, Santos M, Barbadilla A, Quezada-Diaz JE, Hasson E, Fontdevila A (1991) Genetic variance for body size in a natural population of Drosophila buzzatii. Genetics 128:739–750
Santos M, Ruiz A, Barbadilla A, Quezada-Diaz JE, Hasson E, Fontdevila A (1988) The evolutionary history of Drosophila Buzzatii. XIV. Larger flies mate more often in nature. Heredity 61:255–262
Scheiner SM, Goodnight CJ (1984) The comparison of phenotypic plasticity and genetic variation in populations of the grass Danthonia spicata. Evolution 38:845–855
Scheiner SM, Lyman RF (1989) The genetics of phenotypic plasticity I. Heritability. J Evol Biol 2:95–107
Scheiner SM, Caplan RL, Lyman RF (1991) The genetics of phenotypic plasticity. III. Genetic correlations and fluctuating asymmetries. J Evol Biol 4:51–68
Schlichting CD (1986) The evolution of phenotypic plasticity in plants. Annu Rev Ecol Syst 17:667–693
Schlichting CD, Levin DA (1990) Phenotypic plasticity in Phlox. III. Variation among natural populations of P. drummondii. J Evol Biol 3:411–428
Sokal RR, Oden NL, Barker JSF (1987) Spatial structure in Drosophila buzzatii populations: simple and directional spatial autocorrelation. Am Nat 129:122–142
Stalker HD, Carson HL (1947) Morphological variation in natural populations of Drosophila robusta Sturtevant. Evolution 1:237–248
Stalker HD, Carson HL (1948) An altitudinal transect of Drosophila robusta Sturtevant. Evolution 2:295–305
Stalker HD, Carson HL (1949) Seasonal variation in the morphology of Drosophila robusta Sturtevant. Evolution 3:330–343
Starmer WT, Barker JSF (1986) Ecological genetics of the Adh-1 locus of Drosophila buzzatii. Biol J Linn Soc 28:373–385
Starmer WT, Wolf LL (1989) Causes of variation in wing loading among Drosophila species. Biol J Linn Soc 37:247–261
Stearns SC (1989) The evolutionary significance of phenotypic plasticity. BioScience 39:436–445
Stearns S, de Jong G, Newman B (1991) The effects of phenotypic plasticity on genetic correlations. Trends Ecol Evol 6:122–126
Tantawy AO, Mallah GS (1961) Studies on natural populations of Drosophila. I. Heat resistance and geographical variation in Drosophila melanogaster and D. simulans. Evolution 15:1–14
Via S (1984) Th quantitative genetics of polyphagy in an insect herbivore. II. Genetic correlations in larval performance within and among host plants. Evolution 38:896–905
Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39:505–522
Wilkinson GS (1987) Equilibrium analysis of sexual selection in Drosophila melanogaster. Evolution 41:11–21
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall Inc, Englewood Cliffs, New Jersey
Author information
Authors and Affiliations
Additional information
Communicated by G. Wenzel
Rights and permissions
About this article
Cite this article
Thomas, R.H., Barker, J.S.E. Quantitative genetic analysis of the body size and shape of Drosophila buzzatii . Theoret. Appl. Genetics 85, 598–608 (1993). https://doi.org/10.1007/BF00220919
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220919