Abstract
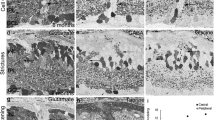
• Background: To study normal quantitative cellular relations and the effect of optic nerve section on neurons, glia and capillaries, morphometry was carried out on 24 whole-mount retinae of 12 rats. • Methods: In the left eye the optic nerve had been sectioned 30 days before death; the right eyes served as controls. Using a cresyl violet stain, cells in the retinal ganglion cell layer were evaluated at three distances from the papilla (1.2, 2.4 and 3.6 mm). • Results: Gradients for density of neurons, glial cells and capillary grid were all within a small range (center: mid:periphery=1.41–1.59: 1.29–1.33: 1.00). For all these distances we found a fairly constant ratio among the three histological parameters: 44.7–46.6 neurons and 2.3–2.6 glial cells were counted per capillary grid square (geometric model for the capillary meshwork). Thirty days after section of the optic nerve the capillary meshwork remained unaffected (96.2 grid squares/mm2 before nerve section vs 94.7 grid squares/mm2 after nerve section) while glial cells had more than doubled (238 vs 498 cells/mm2) and nearly half of all neurons had gone (4371 vs 2244 cell s/mm2). Size characteristics of amacrine cells were similar for all three eccentricities, whereas peripheral retinal ganglion cells tended to be considerably larger than central ones. • Conclusions: Cresyl violet stain can be used to study quantitative changes of neurons, glial cells and capillary grid in the retinal ganglion layer of a single whole-mount retina. There is a remarkable degree of proportionality between the density of these cells over the whole normal retina.
Similar content being viewed by others
References
Allcutt C, Berry M, Sievers J (1984) A quantitative comparison of the reactions of retinal ganglion cells to optic nerve crush in neonatal and adult mice. Dev Brain Res 16: 219–230
Barron KD, Dentinger MP, Krohel G, Easton SK, Mankes R (1986) Qualitative and quantitative ultrastructural observations on retinal ganglion cell layer of rat after intraorbital optic nerve crush. J Neurocytol 15: 345–362
Björklund A, Stenevi U (1985) Neural grafting in the mammalian CNS. Elsevier, Amsterdam
Büssow H (1980) The astrocytes in the optic nerve head and retina of mammals. A special glia for the ganglion cell axons. Cell Tissue Res 206: 367–378
Carpenter P, Sefton AJ, Dreher B, Lim W-L (1986) Role of target tissue in regulating the development of retinal ganglion cells in the albino rat: effects of kainate lesions in the superior colliculus. J Comp Neurol 251: 240–259
Conradi N, Sjöstrand J (1993) A morphometric and stereologic analysis of ganglion cells of the central human retina. Graefe's Arch Clin Exp Ophthalmol 231: 169–174
Cowey A, Perry VH (1979) The projection of the temporal retina in rats, studied by retrograde transport of horseradish peroxidase. Exp Brain Res 35:457–464
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300: 5–25
Dräger UC, Olsen JF (1981) Ganglion cell distribution in the retina of the mouse. Invest Ophthalmol Vis Sci 20: 285–293
Fukuda Y (1977) A three-group classification of rat retinal ganglion cells: histological and physiological studies. Brain Res 119: 327–344
Gellrich N-C, Gellrich M-M, Machtens E (1993) Degeneration nach Sehnervschädigung. Dtsch Z Mund Kiefer Gesichtschir 17: 286–288
Gellrich N-C, Gellrich M-M, Bremerich A (1994) Influence of fetal brain grafts on axotomized retinal ganglion cells. Int J Oral Maxillofac Surg 23: 403–405
Glovinsky Y, Quigley HA, Pease ME (1993) Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 34: 395–400
Goshgarian HG, Koistinen JM, Schmidt ER (1983) Cell death and changes in the retrograde transport of horseradish peroxidase in rubrospinal neurons following spinal cord hemisection in the adult rat. J Comp Neurol 214: 251–257
Hollander H, Bisti S, Maffei L, Hebel R (1984) Electroretinographic responses and retrograde changes of retinal morphology after intracranial optic nerve section. A quantitative analysis in the cat. Exp Brain Res 55: 483–493
Hughes A (1975) A quantitative analysis of the cat retinal ganglion cell topography. J Comp Neurol 163: 107–128
Hughes A, Vaney DI (1980) Coronate cells: displaced amacrines of the rabbit retina? J Comp Neurol 189: 169–189
Hughes WF (1991) Quantitation of ischemic damage in the rat retina. Exp Eye Res 53: 573–582
Humphrey MF, Beazley LD (1985) Retinal ganglion cell death during optic nerve regeneration in the frog Hyla moorei. J Comp Neurol 236: 382–402
James GR (1933) Degeneration of ganglion cell following axonal inury. Arch Ophthalmol 9: 338–343
Kelly JP, Gilbert CD (975) The projections of different morphological types of ganglion cells in the cat retina. J Comp Neurol 163: 65–80
Lierse W (1963) Die Kapillardichte im Wirbeltiergehirn. Acta Anat 54: 1–31
McCall MJ, Robinson SR, Dreher B (1987) Differential retinal growth appears to be the primary factor producing the ganglion cell density gradient in the rat. Neurosci Lett 79: 78–84
Mednick AS, Springer AD (1981) Asymmetric distribution of retinal ganglion cells in goldfish. J Comp Neurol 202:493–504
Mey J, Thanos S (1993) Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res 602: 304–317
Misantone LJ, Gershenbaum M, Murray M (1984) Viability of retinal ganglion cells after optic nerve crush in adult rats. J Neurocytol 13: 449–465
Moriya T, Yamadori T (1993) Correlative study of the morphology and central connections of ipsilaterally projecting retinal ganglion cells in the albino rat. Exp Eye Res 56: 79–83
Neumaier S, Gellrich M-M, Hansen LL (1994) The retinal ganglion cell layer in an eye with drusen in the optic nerve head. Poster, 10th Meeting of International Neuro-Ophthalmological Society, Freiburg. Abstract book
Oppel O (1967) Untersuchungen über die Verteilung und Zahl der retinalen Ganglienzellen beim Menschen. Graefe's Arch Clin Exp Ophthalmol 172: 1–22
Ogden TE (1978) Nerve fibre layer astrocytes of primate retina: morphology, distribution and density. Invest Ophthalmol Vis Sci 17: 499–510
Perry VH (1981) Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience 6: 931–944
Perry VH, Henderson Z, Linden R (1983) Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol 219: 356–368
Potts RA, Dreher B, Bennett MR (1982) The loss of ganglion cells in the developing retina of the rat. Dev Brain Res 3: 481–486
Quigley HA, Dunkelberger GR, Green WR (1989) Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 107: 453–464
Rapaport DH, Stone J (1983) Time course of morphological differentiation of cat retinal ganglion cells: influence on soma size. J Comp Neurol 221: 42–52
Sandell JH (1985) NADPH-diaphorase cells in the mammalian inner retina. J Comp Neurol 238: 466–472
Sievers H, Gronemeyer U, Hansen C, Sievers J (1984) Der Fasciculus opticus als Modell für die Untersuchung von Regenerationsvorgängen im Zentralnervensystem. Fortschr Ophthalmol 81: 164–167
Sievers J, Hausmann B, Unsicker K, Berry M (1987) Fibroblast growth factors promote the survival of adult rat retinal ganglion cells after transection of the optic nerve. Neurosci Lett 76: 157–162
Silva-Araújo A, Salgado-Borges J, Cardoso V, Silva MC, Castro-Correia J, Tavares MA (1993). Changes in the retinal ganglion cell layer and optic nerve of rats exposed neonatally to cocaine. Exp Eye Res 56: 199–206
Sjöstrand J, Conradi N, Klarén L (1994) How many ganglion cells are there to a foveal cone? A stereologic analysis of the quantitative relationship between cone and ganglion cells in one normal human fovea. Graefe's Arch Clin Exp Ophthalmol 232: 432–437
Stone J (1965) A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol 124: 337–352
Stone J (1981) The whole mount handbook — a guide to the preparation and analysis of retinal whole mounts. Maitland, Sydney
Stone J, Dreher Z (1987) Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol 255: 35–49
Thanos S, Bähr M, Barde Y-A, Vanselow J (1989) Survival and axonal elongation of adult rat retinal ganglion cells. Eur J Neurosci 1: 19–26
Thanos S, Rohrbach J-M, Thiel HJ (1991) Postmortem preservation of ganglion cells in the human retina. Retina 11: 318–327
Unoki K, LaVail MM (1994) Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci 35: 907–915
Villegas-Perez MP, Vidal-Sanz M, Bray GM, Aguayo AJ (1988) Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci 8:265–280
Wässle H, Illing R-B (1980) The retinal projection to the superior colliculus in the cat: a quantitative study with HRP. J Comp Neurol 190: 333–356
Wässle H, Levick WR, Cleland BG (1975) The distribution of the alpha type of ganglion cells in the cat's retina. J Comp Neurol 159: 419–438
Wise GN, Dollery CT, Henkind P (1971) The retinal circulation. Harper and Row, New York
Zhang C, Takahashi K, Lam TT, Tso MOM (1994) Effects of basic fibroblast growth factor in retinal ischemia. Invest Ophthalmol Vis Sci 35: 3163–3168
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gellrich, MM., Gellrich, NC. Quantitative relations in the retinal ganglion cell layer of the rat: neurons, glia and capillaries before and after optic nerve section. Graefe's Arch Clin Exp Ophthalmol 234, 315–323 (1996). https://doi.org/10.1007/BF00220707
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220707