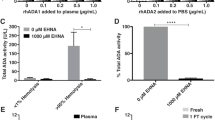
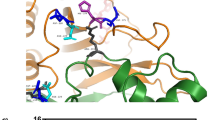
Summary
A deficiency of the enzyme adenosine deaminase is associated with an autosomal recessive form of severe combined immunodeficiency disease in man. The molecular forms of the normal human enzyme have now been well characterized in an effort to better understand the nature of the enzyme defect in affected patients.
In some human tissues adenosine deaminase exists predominantly as a small molecular form while in other tissues a large form composed of adenosine deaminase (small form) and an adenosine deaminase-binding protein predominates. The small form of the enzyme purified to homogeneity by antibody affinity chromatography is a monomer of native molecular weight of 37,600. The adenosine deaminase-binding protein, purified by adenosine deaminase affinity chromatography, appears to be a dimer of native molecular weight 213,000 and contains carbohydrate. Based on direct binding measurements, chemical cross-linking studies and sedimentation equilibrium analyses, small form adenosine deaminase has been shown to combine with purified binding protein in a molar ratio of 2:1 respectively to produce the large form adenosine deaminase.
Reduced, but widely ranging levels of adenosine deaminating activity, have been reported in various tissues of adenosine deaminase deficient patients. Further, the characteristics of this residual enzyme activity have been analyzed immunochemically to substantiate genetic heterogeneity in this disorder.
While many types of immunodeficiency are currently recognized in man, in most cases the molecular defect is unknown. The discovery of a deficiency of the enzyme, adenosine deaminase, ADA, (EC 3.5.4.4), in some patients with severe combined immunodeficiency disease represented an early clue to the pathogenesis of immune dysfunction at the molecular level1-4. Affected patients with markedly reduced levels of ADA exhibit a defect of both cellular and humoral immunity characterized clinically by severe recurrent infections with a fatal outcome if untreated. Attempts to elucidate the nature of the genetic mutation(s) leading to the reduction of ADA activity in these immunodeficient patients have been complicated in part by an incomplete understanding of the nature of ADA in normal tissues. In this review we will consider the structural characteristics of the normal and mutant forms of ADA as they are currently understood.
Similar content being viewed by others
References
Giblett, E. R., Anderson, J. E., Cohen, F., Pollara, B. and Meuwissen, H. J., 1972. Lancet 2, 1067–1069.
Dissing, J. and Knudsen, B., 1972. Lancet 2, 1316.
Parkman, R., Gelfand, E. W., Rosen, F. S., Sanderson, A. and Hirschorn, R., 1975. New Eng. J. Med. 292, 714–719.
Meuwissen, H. J., Pollara, B. and Pickering, R. J. (1975) J. Pediatrics 86, 169–181.
Spencer, N., Hopkinson, D. A., and Harris, H., 1968. Ann. Hum. Genet. 32, 9–14.
Osborne, W. R. A. and Spencer, N., 1973. Biochem. J. 133, 117–123.
Schrader, W. P., Stacy, A. R. and Pollara, B., 1976. J. Biol. Chem. 251, 4026–4032.
Daddona, P. E. and Kelley, W. N., 1977. J. Biol. Chem. 252, 110–115.
Edwards, Y. H., Hopkinson, D. A. and Harris, H., 1971. Ann. Hum. Genet. 35, 207–219.
Nishihara, H., Ishikawa, S., Shinkai, K. and Akedo, H., 1973. Biochem. Biophys. Acta. 302, 429–442.
Hirschhorn, R., Levytska, V., Meuwissen, H. J. and Pollara, B., 1973. Nature New Biol. 246, 200–202.
Creagan, R. P., Tischfield, J. A., Nichols, E. A., and Ruddle, F. H., 1973. Lancet, 1449–1450.
Hirschhorn, R., 1975. J. Clin. Invest. 55, 661–667.
Van der Weyden, M. B. and Kelley, W. N., 1976. J. Biol. Chem. 251, 5448–5456.
Daddona, P. E. and Kelley, W. N., 1979. Biochem. Biophys. Acta in press.
Daddona, P. E. and Kelley, W. N., 1978. J. Biol. Chem. 253, 4617–4623.
Schrader, W. P. and Stacy, A. R. (1977) J. Biol. Chem. 252, 6409–6415.
Swallow, D. M., Evans, L. and Hopkinson, D. A., 1977. Nature 269, 261–262.
De La Haba, G. and Cantoni, G. L., 1959. J. Biol. Chem. 234, 603–608.
Daddona, P. E. and Kelley, W. N., 1979. Unpublished observations.
Koch, G. and Shows, T. B., 1978. Proc. Natl. Acad. Sci. 75,3876–3878.
Van der Weyden, M. B., Buckley, R. H., and Kelley, W. N., 1974. Biochem. Biophys. Res. Commun. 57, 590–595.
Schrader, W. P., Pollara, B. and Meuwissen, H. J., 1978. Proc. Natl. Acad. Sci. 75, 446–450.
Chen, S. H., Scott, C. R. and Swedberg, K. R., 1975. Am. J. Hum. Genet. 27, 46–53.
Hirschhorn, R., Beratis, N. and Rosen, F. S., 1976, Proc. Natl. Acad. Sci. 73, 213–217.
Carson, D. A., Goldblum, R. and Seegmiller, J. E., 1977. J. Immunol. 118, 270–273.
Daddona, P. E., Frohman, M. A. and Kelley, W. N., 1979. J. Clin. Invest. 64, 798–803.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Daddona, P.E., Kelley, W.N. Analysis of normal and mutant forms of human adenosine deaminase — A review. Mol Cell Biochem 29, 91–101 (1980). https://doi.org/10.1007/BF00220303
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00220303