Summary
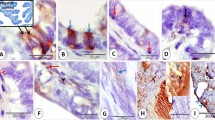
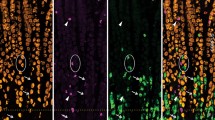
Two-month-old female Swiss mice that had come into estrus were injected intravenously with L-3H-fucose and killed at 5, 15, 40 min, and 4 h after injection. Pieces of the isthmus and of the ampulla of the uterine tubes were processed for light-and electron-microscopic radioautography. Incorporation of 3H-fucose was more intense in the isthmian secretory cells than in the ciliated cells of the ampulla. Electron-microscopic radioautography of the isthmian secretory cells demonstrated that 3H-fucose was incorporated into newly synthesized glycoproteins in the Golgi apparatus from where labelled glycoproteins migrated mainly to secretory granules and apical microvilli. The histochemical technique using ruthenium red confirmed the presence of glycoproteins in the contents of the secretory granules released to the lumen of the uterine tubes as demonstrated by radioautography. Other glycoproteins are transported inside small vesicles and most likely are related to the renewal of the plasma membrane. The role of the secretory glycoproteins in various events of mammalian reproduction is discussed.
Similar content being viewed by others
References
Bennett G, Leblond CP, Haddad A (1974) Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labeled fucose injection into rats. J Cell Biol 60:258–284
Bennett G, Parsons S, Carlet E (1984) Influence of colchicine and vinblastine on the intracellular migration of secretory and membrane glycoproteins: I. Inhibition of glycoprotein migration in various rat cell types as shown by light microscope radioautography after injection of 3H-fucose. Am J Anat 170:521–530
Bronson FH, Dagg CP, Snell GD (1968) Reproduction. In: Biology of the laboratory mouse. Staff of the Jackson Laboratory. Dover Publications Inc, New York, 2nd edition, pp 187–204
Chalkley HW (1943) Method for the quantitative morphologic analysis of tissues. J Natl Cancer Inst 4:47–53
Champlin AK, Dorr DL (1973) Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod 8:491–494
Fox LL, Shivers CA (1975) Immunological evidence for addition of oviductal components to the hamster zona pellucida. Fertil Steril 26:599–608
Fredericsson B (1969) Histochemistry of the oviduct. In: Hafez ESE, Blandau RJ (eds) The mammalian oviduct. The University of Chicago Press, Chicago, pp 311–332
Gibson C, Masters CJ (1970) Oviductal lactate dehydrogenase. J Reprod Fertil 22:157–159
Gutierrez-Gonzalves MG, Stockert JC, Ferrer JM, Tato A (1984) Ruthenium red staining of polyanion containing structures in sections from epoxy-resin embedded tissues. Acta Histochem 74:115–120
Haddad A, Bennett G (1987) Synthesis and migration of 3H-fucose-labeled glycoproteins in the retinal pigment epithelium of albino rats, as visualized by radioautography. Am J Anat 178:259–268
Hamner CE, Fox SB (1969) Biochemistry of oviductal secretions. In: Hafez ESE, Blandau RJ (eds) The mammalian oviduct. Comparative biology and methodology. The University of Chicago Press, Chicago, pp 333–355
Jansen RPS (1984) Endocrine response in the Fallopian tube. Endocrinol Rev 5:525–551
Jansen RPS, Bajpai VK (1983) Periovulatory glycoprotein secretion in the macaque fallopian tube. Am J Obstet Gynec 147:598–698
Kapur RP, Johnson LV (1985) An oviductal fluid glycoprotein associated with ovulated mouse ova and early embryos. Dev Biol 112:89–93
Kopriwa BM (1973) A reliable standardized method for ultrastructural electron microscopic radioautography. Histochemie 37:1–17
Kopriwa BM, Levine GM, Nadler NJ (1984) Assessment of resolution by half distance values for tritium and radioiodine in electron microscopic radioautographs using Ilford L4 emulsion developed by “Solution Physical” or D-19b methods. Histochemistry 80:519–522
Lee MC, Wu TC, Wan YJ, Damjanov I (1983) Pregnancy related changes in the mouse oviduct and uterus revealed by differential binding of fluoresceinated lectins. Histochemistry 79:365–375
Leppi TJ, Kinnison PA (1971) Histochemistry of carbohydrate-rich components in oviductal secretions. Anat Rec 169:367
Luft J (1971) Ruthenium red and violet. II Fine structural localization in animal tissues. Anat Rec 171:369–416
Menghi G, Bondi AM, Accioli D, Vitaioli L (1984a) Glycoconjugates in the reproductive system of female rabbits in physiological estrogenic conditions. Anat Anz 156:125–128
Menghi G, Bondi AM, Accioli D, Materazzi G (1984b) Fine localization of sulphated and non-sulphated glycoconjugates in the rabbit oviduct during the estrous cycle. Acta Histochem 74:121–132
Menghi G, Bondi AM, Materazzi G (1985) Distribution of lectin binding sites in rabbit oviduct. Anat Rec 211:279–284
Michaels JE, Leblond CP (1976) Transport of glycoprotein from Golgi apparatus to cell surface by means of “carrier” vesicles as shown by radioautography of mouse colonic epithelium after injection of 3H-fucose. J Microsc Biol Cell 25:243–248
Moghissi KS (1970) Human fallopian tube fluid I. Protein composition. Fertil Steril 21:821–829
Nilsson O, Reinius S (1969) Light and electron microscopic structure of the oviduct. In: Hafez ESE, Blandau RJ (eds) The mammaliam oviduct. Comparative biology and methodology. The University of Chicago Press, Chicago, pp 57–83
Parlanti IA, Monis B (1975) Histochemistry of the cell surfaces of the mucosa of the oviducts and the uterus of the rat. Changes in puberty, estrous cycle, castration, hormone replacement and pseudopregnancy. Experientia 31:1456–1459
Putti R, Varano L (1979) Histological and histochemical modifications of the uterine and vaginal mucosa of the mouse during the oestrus cycle. Bas Appl Histochem 23:25–37
Shapiro SS, Brown NE, Yard AS (1974) Isolation of an acidic glycoprotein from rabbit oviductal fluid and its association with the egg coating. Biol Reprod 17:281–290
Stone SL, Hamner CE (1975) Biochemistry and Physiology of oviductal secretions. Gynecol Invest 6:234–252
Sutton R, Nancarrow D, Wallace ACL, Rigby NW (1984) Identification of an oestrus-associated glycoprotein in oviductal fluid of the sheep. J Reprod Fertil 72:415–422
Thibault C (1972) Physiology and physiopathology of the Fallopian tube. Int J Fertil 17:1–13
Wu TC, Wan YJ, Damjanov I (1983) Distribution of Bandeirea simplicifolia lectin binding sites in the genital organs of female and male mice. Histochemistry 77:233–241
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teixeira, M.L.S., Haddad, A. Histochemical and radioautographic study of glycoprotein secretion in the epithelium lining the uterine tubes of mice. Cell Tissue Res. 254, 209–216 (1988). https://doi.org/10.1007/BF00220036
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00220036