Summary
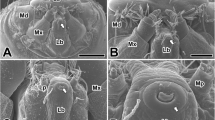
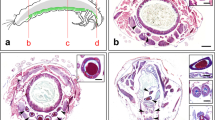
The ultrastructure and amino acid composition of the secreted silk of two species of trichopteran larvae, Pycnopsyche guttifer (Walk.) and Neophylax concinnus McL., were investigated. The spinnerets of these two animals were also examined by scanning electron microscopy. The silk consists of double-stranded, flat ribbons (1–4 μ wide), composed of bundles of 15–25 Å filaments. There are two components of the silk: the fiber proper and a surrounding coat thought to be a silk “gum”. Only the outer coat is positive to the EM PATP technique of Thiery (1967), which indicated the presence of neutral sugars. Amino acid analyses of Pycnopsyche silk show that, like other silks, two predominant amino acids are glycine and serine. Arginine, unexpectedly, is the third most abundant and there are a large number of basic and long side-chain amino acids. X-ray diffraction studies of the silk indicate that it has a less crystalline, more amorphous structure than that of other silks.
Similar content being viewed by others
References
Akai, H.: Ultrastructure of the fibroin in the silk gland of larval Bombyx mori. Exp. Cell Res. 69, 219–223 (1971)
Allegret, P., Fraisse, R.: Étude expérimentale du fonctionnement de l'appareil fileur du Ver à soie, Bombyx mori (L.). C.R. Acad. Sci. (Paris) 249, 165–166 (1959)
Ambrose, E.J., Bamford, C.H., Elliot, A., Hanby, W.E.: Water-soluble silk: an α-protein. Nature (Lond.) 167, 264–265 (1951)
Asano, Y., Sinohara, H.: Amino sugars in various silk fibroins. Naturwissenschaften 55, 345 (1968)
Baier, R.E., Shafrin, E.G., Zisman, W.A.: Adhesion: mechanisms that assist or impede it. Science 162, 1360–1368 (1968)
Bamford, C.H., Elliot, A., Hanby, W.E.: Synthetic polypeptides, pp. 369–408. New York: Academic Press 1956
Bergen, W. von, Knauss, W.: Textile fiber atlas. I. Wool. Rayon Tex. Mon. 21, 17–20 (1940)
Bergmann, W.: Facts and theories concerning the formation of natural silk. Textile Res. J. 9, 329–342 (1939)
Branch, H.E.: A contribution to the knowledge of the internal anatomy of Trichoptera. Ann. ent. Soc. Amer. 15, 256–275 (1922)
Chen, J.L., Cyr, G.N.: Compositions producing adhesion through hydration. In: Adhesion in biological systems, ed. by R.S. Manly, Chap. 10. New York: Academic Press 1970
DeWilde, J., Some physical properties of the spinning threads of Aranea diadema, L. Arch. néerl. Physiol. 27, 118–132 (1943)
Dobb, M.G., Fraser, R.D.B., McRae, T.P.: The fine structure of silk fibroin. J. Cell Biol. 32, 289–295 (1967)
Engster, M.: Studies on silk secretion in the Trichoptera (F. Limnephilidae). I. Histology, histochemistry, and ultrastructure of the silk glands. J. Morph. (in press)
Fraser, R.D.B., MacRae, T.P.: Conformation in fibrous proteins and related synthetic polypeptides. New York: Academic Press 1973
Glascow, J.P.: Internal anatomy of a caddis (Hydropsyche colonica). Quart. J. micr. Sci. 79, 151–179 (1936)
Glatz, L.: Der Spinnapparat haplogyner Spinnen (Arachnida, Araneae). Z. Morphol. Tiere 72, 1–25 (1972)
Gottel L., Mammi, M., Pezzin, G.: Scanning electron microscope observations on elastin. Conn. Tiss. Res. 1, 61–67 (1972)
Gross, J., Schmitt, F.O.: The structure of human skin collagen as studied with the electron microscope. J. exp. Med. 88, 555–569 (1948)
Hall, F.K.: Wood pulp. Sci. Amer. 230, 52–62 (1974)
Hazan, A., Gertler, A., Tahori, A.S., Gerson, U.: Spider mite webbing —III. Solubilization and amino acid composition of the silk protein. Comp. Biochem. Physiol. 51B, 457–462 (1975)
Howitt, F.O.: Bibliography of the technical literature on silk. London: Hutchinson 1947
Hunt, S.: Polysaccharide-protein complexes in invertebrates. New York: Academic Press 1970
Iizuka, E.:Mechanism of fiber formation by the silkworm, Bombyx mori. Biorheology 3, 141–152 (1966)
Lenormat, H.: Infra-red spectra and structure of the proteins of the silk glands. Trans. Faraday Soc. 52, 549–553 (1956)
Lloyd, J.T.: The biology of North American caddis fly larvae. Bull. Lloyd Libr., Entomol. Ser. 1, 21, 1–124 (1921)
Lucas, F., Rudall, K.M.: Extracellular fibrous proteins: the silks. In: Comprehensive biochemistry, vol. 26B, ed. by M. Florkin and E. Stotz, pp. 475–558. New York: Elsevier Publishing Co. 1968
Lucas, F., Shaw, J.T.B., Smith, S.G.: The silk fibroins. Adv. Protein Chem. 13, 107–242 (1958)
Lucas, F., Shaw, J.T.B., Smith, S.G.: Comparative studies of fibroin. I. The amino acid composition of various fibroins and its significance in relation to their crystal structure and taxonomy. J. molec. Biol. 2, 339–349 (1960)
Lucas, R.: Beiträge zur Kenntnis der Mundwerkzeuge der Trichoptera. Arch. Naturgesch. 59, 285–330 (1893)
Mercer, E.H.: Formation of the silk fibre by the silkworm. Nature (Lond.) 168, 792–793 (1951)
Meyer, K.H., Jeannerat, J.: Les proprietés des polymères en solution. XI. Sur la formation du fil de soie à partir du continu liquide de la glande. Helv. chim. Acta 22, 22–30 (1939)
Peters, H.M.:Über den Spinnapparat von Nephila madagascariensis (Radnetzspinnen, Fam. Argiopidae). Z. Naturforsch. 10b, 395–404 (1955)
Ramsden, W.: Coagulation by shearing and by freezing. Nature (Lond.) 142, 1120–1121 (1938)
Raven, D.J., Earland, C., Little, M.: Occurrence of dityrosine in tussah silk fibroin and keratin. Biochem. biophys.Acta (Amst.) 251, 96–97 (1971)
Rudall, K.M.: Silk and other cocoon proteins. Comp. Biochem. 4, 397–433 (1962)
Rudall, K.M., Kenchington, W.: Arthropod silks: the problem of fibrous proteins in animal tissues. Ann. Rev. Entomol. 16, 73–96 (1971)
Seifter, S., Gallop, P.M.: The structure proteins. In: The proteins: Composition, structure, and function, 2nd ed., ed. by A. Neurath, Vol. IV. New York: Academic Press 1963
Senti, F.C.: Structure of protein fibers. Amer. Dyestuff Reporter 36, 230–237 (1947)
Sinohara, Y., Asano, Y.: Carbohydrate content of fibroin and sericin of the silkworm, Bombyx mor. J. Biochem. 62, 129–130 (1967)
Thiery, J.P.: de]Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J. Micr. (Paris) 6, 987–1018 (1067)
Tindall, A.R.: The larval case of Triaenodes bicolor Curtis (Trichoptera: Leptoceridae). Proc. roy. ent. Soc. London A 35, 93–96 (1960)
Author information
Authors and Affiliations
Additional information
Submitted to the Department of Biological Sciences of the State University of New York at Albany in partial fulfillment of the requirements for the degree of Doctor of Philosophy
Acknowledgements. This study was supported in part by a National Institutes of Health Graduate Student Traineeship grant # GM-02014. The author would like to express sincere gratitude to Dr. Stephen Brown for his encouragement and help during the course of this study. I would also like to thank Dr. Curtis Hemenway and Mr. Douglas Halgren of Dudley Observatory for the use of their scanning electron microscope as well as Dr. Helen Ghiradella and Mr. William Radigan for help with the scanning electron microscopy. I owe special appreciation to Dr. Y. Myer of the Chemistry Department of SUNYA for doing an amino acid analysis of the silk and to Dr. K.M. Rudall of the University of Leeds for doing the X-ray diffraction studies of the silk samples
Rights and permissions
About this article
Cite this article
Engster, M.S. Studies on silk secretion in the trichoptera (F. Limnephilidae). Cell Tissue Res. 169, 77–92 (1976). https://doi.org/10.1007/BF00219309
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00219309