Summary
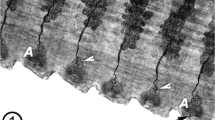
The ultrastructure of microtriches of the rat tapeworm, Hymenolepis diminuta, was examined with a number of electron-microscopic techniques. Fixatives containing different buffers, non-ionic detergents, chelators, tannic acid and various concentrations of aldehydes were tested for ability to stabilize cytoskeletal components while extracting background material. These methods revealed features unique to these specialized microvilli, and permitted construction of a detailed model of microthrix architecture. The microtriches of H. diminuta are comprised of a microfilament-containing base, a dense cap and a complex junctional region between the base and cap. The microfilaments of the base are contiguous distally with a tubular structure (the junctional tubule) within the junctional region; proximally, the microfilaments end abruptly: a terminal web appears to be absent. A beveled bilayered cylinder of dense material (the core tunic) encircles the microfilamentous core. The core tunics and junctional tubules of the microtriches are specifically and uniformly aligned along the strobila. Microtriches therefore can be distinguished from other microvilli (e.g., those of enterocyte brush borders) by their complex ultrastructure and precise orientation upon the cytoplasmic surface.
Similar content being viewed by others
References
Berger J, Mettrick DF (1971) Microtrichial polymophism among hymenolepid tapeworms as seen by scanning electron microscopy. Trans Am Microsc Soc 90:393–403
Carraway CAC, Jung G, Hinkley RE, Carraway KL (1985) Isolation of microvillar microfilaments and associated transmembrane complex from ascites tumor cell microvilli. Exp Cell Res 157:71
Coggins JR (1980) Tegument and apical end organ fine structure in the metacestode and adult Proteocephalus ambloplitis. Int J Parasitol 10:409–418
Cohen C, Reinhardt B, Castellani L, Norton P, Stirewalt M (1982) Schistosome surface spines are “crystals” of actin. J Cell Biol 95:987–988
DeRosier DJ, Tilney LG (1984) The form and function of actin: A product of its unique design. In: Shay JW (ed) Cell and muscle motility, Vol. 5, The cytoskeleton. Plenum Press, New York, pp 139–169
Englekirk PG, Williams JF (1983) Taenia taeniaeformis (Cestoda) in the rat: Ultrastructure of the host-parasite interface on days 8 to 22 postinfection. J Parasitol 69:828–837
Grammeltvedt AF (1973) Differentiation of the tegument and associated structures in Diphyllobothrium dendriticum Nitsch (1824) (Cestoda: Pseudophyllidea). Int J Parasitol 3:321–327
Hayunga EG, Mackiewicz JS (1975) An electron microscope study of the tegument of Hunterella nodulosa. Mackiewicz and McCrae, 1962 (Cestoidea: Caryophyllidea). Int J Parasitol 5:309–319
Hess E, Guggenheim R (1977) A study of the microtriches and sensory processes of the tetrathyridium of Mesocestoides corti Hoeplli, 1925, by transmission and scanning electron microscopy. Z Parasitenkd 53:189–199
Jha RK, Smyth JD (1969) Echinococcus granulosus: Ultrastructure of the microtriches. Exp Parasitol 25:232–244
Kenny AJ, Booth AG (1978) Microvilli: Their ultrastructure, enzymology and molecular organization. In: Campbell PN, Aldridge WN (eds) Essays in biochemistry, Vol. 14, Academic Press, New York
Lumsden RD (1966) Cytological studies on the absorptive surfaces of cestodes. I. The fine structure of the strobilar integument. Z Parasitenkd 27:355–382
Lumsden RD (1975) Surface ultrastructure and cytochemistry of parasitic helminths. Exp Parasitol 37:267–339
Lumsden RD, Specian R (1980) The morphology, histology, and fine structure of the adult stage of the cyclophyllidean tapeworm Hymenolepis diminuta. In: Arai HP (ed) Biology of the tapeworm Hymenolepis diminuta. Academic Press, New York, pp 157–280
Lumsden RD, Voge M, Sogandares-Bernal F (1982) the metacestode tegument: Fine structure, development, topochemistry and interactions with the host. In: Flisser A, Willms K, Laclette JP, Larralde C, Ridaura C, Beltran F (eds) Cysticercosis ⇆resent state of knowledge and perspectives. Academic Press, New York, pp 307–361
MacKinnon BM, Burt MD (1983) Polymorphism of microtriches in the cysticercoid of Ophryocotyle insignis Lonnberg, 1890 from the limpet Patella vulgata. Can J Zool 61:1062–1070
Marchiondo AA, Andersen FL (1983) Fine structure and freezeetch study of the protoscolex tegument of Echinococcus multilocularis (Cestoda). J Parasitol 69:709–718
Maupin P, Pollard TD (1983) Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol 96:51–62
Mount PM (1970) Histogenesis of the rostellar hooks of Taenia crassiceps (Zeder, 1800) (Cestoda). J Parasitol 56:947–961
Oaks JA, Knowles WJ, Cain GD (1977) A simple method of obtaining an enriched fraction of tegumental brush border from Hymenolepis diminuta. J Parasitol 63:476–485
Pearson AGM, Fincham AG, Waters H, Bundy DAP (1985) Diferences in composition between Fasciola hepatica spines and cestode hooks. Comp Biochem Physiol 81B:373–376
Read CP (1955) Intestinal physiology of the host-parasite relationship. In: Cole W (ed) Some physiological aspects and consequences of parasitism. Rutgers University Press, New Brunswick, pp 27–43
Read CP, Rothman A, Simmons J (1963) Studies on membrane transport, with special reference to parasite-host integration. Ann NY Acad Sci 113:154–205
Richards KS, Arme C (1981) Observations on the microtriches and stages in their development and emergence in Caryophyllaeus laticeps (Caryophyllidea: Cestoda). Int J Parasitol 11:369–375
Rothman AH (1963) Electron microscopic studies of tapeworms: the surface structures of Hymenolepis diminuta (Rudolphi, 1819) Blanchard, 1891. Trans Am Microsc Soc 82:22–29
Schliwa M, van Blerkom J (1981) Structural interaction of cytoskeletal components. J Cell Biol 90:222–235
Thompson RCA, Hayton AR, Jue Sue LP (1980) An ultrastructural study of the microtriches of adult Proteocephalus tidswelli (Cestoda: Proteocephalidea). Z Parasitenkd 64:95–111
Threadgold LT (1984) Parasitic platyhelminthes. In: Bereiter-Hahn J, Matoltsy AG, Sylvia Richards K (eds) Biology of the integument. 1. Invertebrates. Springer, Berlin Heidelberg New York Tokyo, pp 132–191
Ubelaker JE, Allison VF, Specian RD (1973) Surface topography of Hymenolepis diminuta by scanning electron microscopy. J Parasitol 59:667–671
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holy, J.M., Oaks, J.A. Ultrastructure of the tegumental microvilli (microtriches) of Hymenolepis diminuta . Cell Tissue Res. 244, 457–466 (1986). https://doi.org/10.1007/BF00219222
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219222