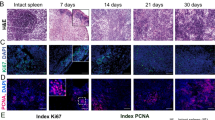
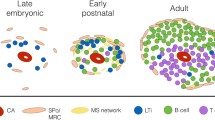
Summary
Regeneration of splenic tissue after autologous subcutaneous implantation provides a useful model for studying the development of splenic tissue. The development of the various non-lymphoid cells of the white pulp in the rat is described. It appears that regeneration of the implants is initiated by ingrowing vessels and a newly formed reticulum, which forms the microenvironment for the homing lymphocytes. Marginal metallophils are found at their characteristic location at the inner border of the marginal sinus five weeks after implantation. Trapping of antigen-antibody complexes reappears when the first primary follicles can be recognized.
Similar content being viewed by others
References
Brachet J (1953) The use of basic dyes and ribonuclease for the cytochemical detection of ribonucleic acid. Q J Micr Sci 94:1–10
Calder RM (1939) Autoplastic splenic grafts: their use in the study of the growth of splenic tissue. J Pathol 49:351
Cameron GR, Khyyu-Sin Rhee (1959) Compensatory hypertrophy of the spleen: a study of splenic growth. J Pathol Bact 78:335–349
Chen LL, Adams JC, Steinman RM (1978a) Anatomy of germinal centers in mouse spleen with special reference to follicular dendritic cells. J Cell Biol 77:148–164
Chen LL, Frank AM, Adams JC, Steinman RM (1978b) Distribution of horseradish peroxidase (HRP) anti-HRP immune complexes in mouse spleen with special reference to follicular dendritic cells. J Cell Biol 79(1):184–199
Davis BJ, Ornstein L (1959) High resolution enzyme localization with a new diazo reagent “Hexazonium pararosaniline”. J Histochem Cytochem 7:297–298
Didukh MS (1967) Regeneration of the lymph nodes after transplantation. Byull Eksperim Biol i Med 12:84–88
Dukor P, Miller JFAP, House W, Allman V, (1965) Regeneration of thymic grafts; histological and cytological aspects. Transplant 3:639–668
Dumont AE (1978) Filtration of circulating particles in splenic autotransplants. Anat Rec 192:261–268
Eikelenboom P (1978) Dendritic cells in rat spleen follicles. Cell Tissue Res 190:79–87
Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasuk AF, Keiliss-Borok IV (1974) Stromal cells responsible for transferring the microenvironment of the haemopoietic tissue. Transplant 17:331–340
Green I (1964) The regeneration of F1 host cell spleen and thymus at ectopic sites in F1 animals induced by implantation of parental spleen and thymus. J Exp Med 119:581–592
Groscurth P (1980) Non-lymphatic cells in the lymph node cortex of the mouse. II. Postnatal development of the interdigitating cells and the dendritic reticular cells. Pathol Res Pract 169:235–254
Heuserman U, Zurborn K-H, Schroeder L, Stutte HJ (1980) The origin of the dendritic reticulum cell. Cell Tissue Res 209:279–294
Kamperdijk EAW, Raaijmakers EM, de Leeuw JHS, Hoefsmit EChM (1978) Lymphnode macrophages and reticulum cells in the immune response. I. The primary response to paratyphoid vaccin. Cell Tissue Res 192(1): 1–23
Katsura S, Satodate R, Fukuda Y, Terui Y (1964) Morphological studies on the reticuloendothelial system in tumor bearing hosts. I. Silver impregnation for reticuloendothelial cells. J Iwate Med Ass 16:232–236
Kuralesova AI (1974) Histogenetic interactions between stromal and hemogenetic cells in heterotopic spleen grafts. Sov J Dev Biol 4(6):530–534
Likhite VV (1978) Protection against fulminant sepsis in splenectomized mice by implantation of autochtonous splenic tissue. Exp Hematol 6(5):433–440
Lowry OH, Rosebrough NJ, Farr AL, Randail RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Manley OT, Marine D (1917) The transplantation of splenic tissue into the subcutaneous fascia of the abdomen in rabbits. J Exp Med XXV:619–627
Marshall AHE (1956) Outline of the cytology and pathology of the reticular tissue. Oliver and Boyd, Edinburgh
McFadden KD (1968) Some reticuloendothelial cells in the white pulp region of the rat spleen. J Reticuloendothel Soc 5:385–398
Nossal GJV, Ada GL (1971) Antigens, lymphoid cells and the immune respons. Dixon jr FJ, Kunkel HG (eds) Academic Press, New York London
Nossal GJV, Abbot A, Mitchell J, Lummus Z (1968) Antigens in immunity XV Ultrastructural features of antigen capture in primary and secondary follicles. J Exp Med 127:277–289
Pabst R, Reilmann H (1980) Regeneration of heterotopically transplanted autologous splenic tissue. Cell Tissue Res 209:137–143
Pearse AGE (1968) Histochemistry. Theoretical and applied Vol I, 3rd ed, Churchill, Edinburgh Livingston
Pearse AGE (1972) Histochemistry. Theoretical and applied Vol II, 3rd ed, Churchill, Edinburgh Livingston
Perla D (1930) Studies on Bartonella muris anemia of albinorats. III. The protective effect of autoplasitc transplants on the Bartonella muris anemia of splenectomized rats. J Exp Med 52:131–143
Pettersen JC, Borgen DF, Graupner KC (1967) A morphological and histochemical study of the primary and secondary immune response in the rat spleen. Am J Anat 121:305–318
Romeis B (1948) Mikroskopische Technik 15, verbesserte Auflage, Leibniz Verlag München p 356
Rooijen N van, Streefkerk JG (1976) Autoradiography and immunohistoperoxidase techniques applied to the same tissue sections. J Immunol Methods 10:379–383
Rozing J, Brons NHC, Ewijk W v, Benner R (1978) B lymphocyte differentiation in lethally irradiated and reconstituted mice: a histological study using immunofluorescent detection of B lymphocytes. Cell Tissue Res 189:19–30
Schwartz AD, Dadash-Zadeh M, Goldstein R, Luck S, Conway JJ (1977) Antibody response to intravenous immunization following splenic tissue autotransplantation in Sprague-Dawley rats. Blood 49(5): 779–783
Shafir M, Deysine M, Bramis J, Acker P, Aufres AH (1977) Immunologic function of subcutaneous splenic implants in mice. Surg Fossum 28:335–337
Silverberg M (1977) Behaviour of transplanted spleen. Arch Pathol Lab Med 20:216–221
Snook T (1964) Studies on the perifollicular region of the rat spleen. Anat Rec 148:149–160
De Sousa HAB (1969) Reticulum arrangement related to the behaviour of cell population in the mouse lymphnode. In: Fiori-Donati, (ed) Lymphatic tissue and germinal centers in immune response, Plenum Press, New York
Streefkerk JG, Van der Ploeg M, Van Minnen J, Touw JJA, Boorsma DM (1976) Immunohistoperoxidase reaction on HRP-coupled agarose beads for titration of anti-HRP antiserum. First internat symp on immuno-enzymatic techniques INSERM Symp no 2, Feldmann (ed), pp 211–224
Stutte HJ, Parwaresch MR, Rossius H (1974) Über die Regeneration von Milz-Autotransplantaten beim Kaninchen: Ferment-histochemische Untersuchungen. Res Exp Med 163:79–94
Tavassoli M, Ratzan RJ, Croby WH (1973) Studies on regeneration of heterotopic splenic autotransplants. Blood 41:701–709
Trentin JJ (1971) Determination of bone marrow stem cell differentiation by stromal haemopoietic inductive microenvironment (HIM). Am J Pathol 65:621
Veerman AJP (1975) The postnatal development of the white pulp in the rat spleen and the onset of immunocompetence against a thymus independent and a thymus dependent antigen. Z Immunitaets Forsch Immunobiol 150:45–59
Veerman AJP, Ewijk W v (1975) White pulp compartments in the spleen of rats and mice. A light and electronmicroscopical study of lymphoid and non-lymphoid cell types in T and B cell areas. Cell Tissue Res 156:417–441
Weiss L (1972) The cells and tissues of the immune system. Structure, functions, interactions. In: Osler AG, Weiss L (eds) Foundation of immunology series. Prentice-Hall Inc Englewood Cliffs, New Yersey
Williams AM, Nossal GJV (1966) Ontogeny of the immune response. I. The development of the follicular antigen trapping mechanism. J Exp Med 124:47–56
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dijkstra, C.D., Langevoort, H.L. Regeneration of splenic tissue after autologous subcutaneous implantation: Development of non-lymphoid cells in the white pulp of the rat spleen. Cell Tissue Res. 222, 69–79 (1982). https://doi.org/10.1007/BF00218289
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218289