Summary
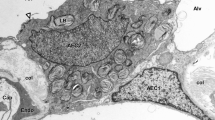
A morphometric analysis was made of alterations in serous cell structure induced by adrenergic and cholinergic agonists. Ferret tracheal rings were exposed for 30 min in vitro to one of the following agonists: phenylephrine, terbutaline, or methacholine (all at 10−5 M). Controls were incubated similarly in medium containing no drugs or medium containing both the agonist and an excess of the appropriate antagonist (phentolamine, propranolol or atropine, all at 10−4 M).
Electron microscopic observation and stereological analysis of the incubated samples revealed that the volume density of serous cell granules in controls (0.30 ± 0.02, mean ± SE, n = 4) was significantly reduced by phenylephrine (0.19 ± 0.03, n = 4) and methacholine (0.17 ± 0.01, n = 4), but not by terbutaline (0.27 ± 0.04, n = 4). The presence of antagonists in the medium prevented the observed changes (phenylephrine/phentolamine: 0.29 ± 0.03, n = 3 and methacholine/atropine: 0.33 ± 0.06, n = 3). In addition, the volume density of intracellular vacuoles in controls (0.02 ± 0.005, n = 4) was increased in response to methacholine stimulation (0.12 + 0.05, n = 4), but not in response to the other agonists. This effect was blocked by atropine (0.01 ± 0.00, n = 3).
We conclude that serous-cell granules are discharged by both alpha-adrenergic and cholinergic, but not beta-adrenergic stimulation. In addition, cholinergic stimulation evokes the formation of intracellular vacuoles, a possible indication of active ion and water transport.
Similar content being viewed by others
References
Amsterdam A, Chad I, Schramm M (1969) Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol 41:753–773
Batzri S, Amsterdam A, Selinger Z, Ohad I, Schramm M (1971) Epinephrine-induced vacuole formation in parotid gland cells and its independence of the secretory process. Proc Natl Acad Sci USA 68:121–123
Boat T, Kleinerman J (1975) Human respiratory tract secretions. II. Effect of cholinergic and adrenergic agents on in vitro release of protein and mucous glycoprotein Chest 67:32s-34s
Bogart BI (1975) Secretory dynamics of the rat submandibular gland: an ultrastructural and cytochemical study of the isoproterenol-induced secretory cycle. J Ultrastruct Res 52:139–155
Chakrin L, Baker A, Christian P, Wardell J (1973) Effects of cholinergic stimulation on the release of macromolecules by canine trachea in vitro. Am Rev Respir Dis 108:69–76
Cope GH, Williams MA (1980) Restitution of granule stores in the rabbit parotid gland after isoprenaline-induced secretion. A stereological analysis of volume parameters. Cell Tissue Res 209:315–327
Cope GH, Williams MA (1973) Exocrine secretion in the parotid gland: a stereological analysis at the electron microscopic level of the zymogen granule content before and after isoprenaline-induced degranulation. J Anat 116:269–284
Davis B, Chinn R, Graf P, Popovac D, Nadel J (1979) Effect of phenylephrine and superior laryngeal nerve stimulation on submucosal gland secretion in canine trachea in vivo. Clin Res 27:55A
Diamond JM, Bossert WH (1967) Standing gradient osmotic flow: a mechanism for coupling of water and solute transport in epithelia. J Gen Physiol 50:2061–2083
Florey H, Carleton H, Wells AM (1932) Mucus secretion in the trachea. Br J Exp Pathol 13:269–284
Gallagher J, Kent P, Passatore M, Phipps R, Richardson P (1975) The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond Biol 192:49–76
Ganote C, Grantham J, Moses H, Burg M, Orloff J (1968) Ultrastructural studies of vasopressin effect on isolated perfused renal collecting tubules of the rabbit. J Cell Biol 36:355–367
Garrett JR, Thulin A, Kidd A (1978) Variations in parasympathetic secretory and structural responses resulting from differences in teh pre-stimulation state of parotid acini in rats: some factors affecting watery vacuolization. Cell Tissue Res 188:235–250
Garrett JR, Thulin A (1975) Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res 159:179–193
German VF, Preiss J, Nadel JA (1981) Alpha and beta adrenergic stimulated release of 3H-threonine and 35S-sulfate labelled glycoproteins from isolated tracheal segments. Clin Res 29:99A
Jamieson JD, Palade GE (1971) Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol 50:135–158
Leslie BA, Putney JW, Sherman JM (1976) Alpha-adrenergic, beta-adrenergic and cholinergic mechanisms for amylase secretion by rat parotid gland in vitro. J Physiol 260:351–370
Marin MG, Davis B, Nadel JA (1976) Effect of acetylcholine on Cl− and Na+ fluxes across dog tracheal epithelium in vitro. Am J Physiol 231:1546–1549
Meyrick B, Reid L (1970) Ultrastructure of cells in the human bronchial submucosal glands. J Anat 107:281–299
Murlas C, Nadel JA, Basbaum CB (1980) A morphometric analysis of the autonomic innervation of cat tracheal glands. J Auton Nerv Syst 2:23–37
Phipps RJ, Nadel JA, Davis B (1980) Effect of alpha-adrenergic stimulation on mucus secretion and on ion transport in cat trachea in vitro. Am Rev Respir Dis 121:359–365
Putney JW, Van de Walle CM, Leslie BA (1978) Stimulus-secretion coupling in the rat lacrimal gland. Am J Physiol 235:C188-C198
Schramm M, Selinger Z (1974) The function of alpha and beta adrenergic receptors and a cholinergic receptor in the secretory cell of rat parotid gland. In: Ceccarelli B, Clementi F, Melddolesi J (ed) Advances in Cyto-pharmacology. Raven Press, New York, Vol 2, p 29
Schramm M, Selinger Z (1975) The functions of cAMP and calcium as alternative second messengers in parotid gland and pancreas. J Cyclic Nucleotide Res 1:181–192
Silva D, Ross G (1974) Ultrastructural and fluorescence histochemical studies on the innervation of the tracheabronchial muscle of normal cats and cats treated with 6-hydroxydopamine. J Ultrastruct Res 47:310–328
Smulders AP, Tormey JM, Wright EM (1972) The effect of osmotically induced water flow on the permeability and ultrastructure of the rabbit gallbladder. J Membr Biol 7:164–197
Specian R, Neutra M (1980) Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol 85:626–640
Sturgess J, Reid L (1972) An organ culture study of the effects of drugs on the secretory activity of the human bronchial submucosal gland. Clin Sci 43:533–543
Tom-Moy M, Basbaum C, Nadel JA (1981) Effects of cholinergic and adrenergic stimulation on lysozyme secretion in ferret trachea. Fed Proc 40:622
Tapp RC (1969) The mechanism of watery vacuolization in the acinar cells of the submandibular gland. J Cell Sci 4:55–70
Ueki I, German V, Nadel J (1980) Micropipette measurement of airway submucosal gland secretion: autonomic effects. Am Rev Respir Dis 121:351–357
Weibel ER (1973) Stereological techniques for electron microscopic morphometry. In: Hayat MA (ed) Principles and techniques of electron microscopy. Van Nostrand Reinhold Co, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Basbaum, C.B., Ueki, I., Brezina, L. et al. Tracheal submucosal gland serous cells stimulated in vitro with adrenergic and cholinergic agonists A morphometric study. Cell Tissue Res. 220, 481–498 (1981). https://doi.org/10.1007/BF00216752
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216752