Summary
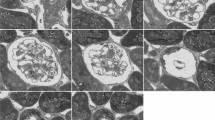
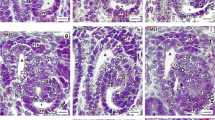
In freeze-fracture replicas of the entire cross-fractured mesonephros of 18 day rabbit embryos the basolateral and luminal cell faces of the different nephron segments were studied and compared with their metanephric counterparts. In the proximal tubule, the shallow zonula occludens exhibited only 1–2 strands and resembled the corresponding metanephric zonula, a very “leaky” type, which was found with a considerable paracellular flow component in sites of isotonic reabsorption. Gap junctions were restricted to the proximal tubule and were seen more frequently in its terminal segment. The distal tubule harboured two types of tight junctions. The most common type, a band of 5–8 closely parallel strands, matched the zonula occludens of the metanephric straight distal tubule. The observed particle density of the basolateral membrane (2,500±328/μm2) was less than that of the proximal tubule (2,642±306). In addition, the collecting tubule exhibited a zonula occludens of the “tight” variety similar to that which occurred in the metanephric collecting duct. Rod-shaped particles of the luminal membrane were mainly concentrated in some of the intercalated cells but also had developed on principal cells, and occasionally, in the distal tubule. The Wolffian duct, with a deep “tight” zonula occludens, had an obviously rather inactive epithelium with no conspicuous transport-linked membrane specializations.
Similar content being viewed by others
References
Bindslev N, Tormey J McD, Wright EM (1974) The effects of electrical and osmotic gradients on lateral intercellular spaces and membrane conductance in a low resistance epithelium. J Mebr Biol 19:357–380
Burg MG, Green N (1973) Function of the thick ascending limb of Henle's loop. Am J Physiol 244:659–668
Claude Ph (1978) Morphological factors influencing transepithelial permeability: A model for the resistance of the zonula occludens. J Membr Biol 39:219–232
Claude Ph, Goodenough DA (1973) Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol 58:390–400
Davies J, Routh JI (1957) Composition of the foetal fluids of the rabbit. J Embryol Exp Morphol 5:32–39
Di Bona DR, Mills JW (1979) Distribution of Na+-pump sites in transporting epithelia. Fed Proc 38:134–143
Ernst SA, Dodsen WC, Karnaky KJ (1980) Structural diversity of occluding junctions in the lowresistance chloride-secreting opercular epithelium of seawater-adapted killifish (Fundulus hetero- clitus). J Cell Biol 87:488–497
Farquhar MG, Palade GE (1963) Junctional complexes in various epithelia. J Cell Biol 17:375–412
Frederiksen O, Møllgard K, Rostgaard J (1979) Lack of correlation between transepithelial transport capacity and paracellular pathway ultrastructure in alcian blue-treated rabbit gallbladders. J Cell Biol 83:383–393
Frömter E (1972) The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol 8:259–301
Humbert F, Pricam C, Perrelet A, Orci L (1975) Specific plasma membrane differentiations in the cells of the kidney collecting tubule. J Ultrastr Res 52:13–20
Humbert F, Grandchamp A, Pricam C, Perrelet A, Orci L (1976) Morphological changes in tight junctions of Necturus maculosus proximal tubules undergoing saline diuresis. J Cell Biol 69:90–96
Huss RE, Marsh DJ (1975) A model of NaCl and water flow through paracellular pathways of renal proximal tubules. J Membr Biol 23:305–347
Kaissling B, Kriz W (1979) Structural analysis of the rabbit kidney. Adv Anat Embryol 56:1–121
Kriz W, Schiller A, Kaissling B, Taugner R (1980) Comparative and functional aspects of the thin limb ultrastructure. In: Maunsbach AB, Ølsen TS, Christensen EI (eds) Functional ultrastructure of the kidney. Academic Press, London 17:239–250
Kriz W, Schiller A, Taugner R (1981) Freeze-fracture studies on the thin limbs of Henle's loop in Psammomys obesus. Am J Anat (in press)
Kühn K, Reale E (1975) Junctional complexes of the tubular cells in the human kidney as revealed with freeze-fracture. Cell Tissue Res 160:193–205
Lewis FT (1920) The course of the Wolffian tubules in mammalian embryos. Am J Anat 26:423–435
Machen TE, Erlij D, Wooding FBP (1972) Permeable Junctional complexes. J Cell Biol 54:302–312
Malnic G, Giebisch G (1972) Some electrical properties of distal tubular epithelium in the rat. Am J Physiol 223:797–808
Martínez-Palomo A, Erlij D (1975) Structure of tight junctions in epithelia with different permeability. Proc Natl Acad Sci USA 72:4487–4491
Martínez-Palomo A, Meza I, Beaty G, Cereijido M (1980) Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol 87:736–745
Meldolesi J, Castiglione G, Parma R, Nassivera N, De Camilli P (1978) Ca++ dependent disassembly and reassembly of occluding junctions in guinea pig pancreatic acinar cells. Effect of drugs. J Cell Biol 79:156–172
Meza I, Iberra G, Sabanero M, Martínez-Paloma A, Cereijido M (1980) Occluding junctions and cytoskeletal components in a cultured transporting epthelium. J Cell Biol 87:746–754
Minuth M (1980) Die Differenzierung der Zell-Zellverbindungen und die Entstehung des juxtaglomerulären Apparates in der reifenden Säugerniere: Diss. Köln
Oschmann JL (1978) Morphological correlates of transport. In: Giebisch G, Tosteson DC, Ussing HH (eds) Membrane transport in biology. Springer Verlag, Berlin Heidelberg New York, Vol III, chapt 3, pp 67–93
Peek W, Shivers RR, McMillan DB (1977) Freeze-fracture analysis of Junctional complexes in the nephron of the garter snake, Thamnophis sirtalis. Cell Tissue Res 179:441–451
Pricam C, Humbert F, Perrelet A, Orci L (1974) A freeze-etch study of the tight junctions of the rat kidney tubules. Lab Invest 30:286–291
Riddle CV, Ernst SA (1979) Structural simplicity of the zonula occludens in the electrolyte secreting epithelium of the avian salt gland. J Membr Biol 45:21–35
Roesinger B, Schiller A, Taugner L (1978) A freeze-fracture study of tight junctions in the pars convoluta and pars recta of the renal proximal tubule. Cell Tissue Res 186:121–133
Sackin H, Boulpaep EL (1975) Models for coupling of salt and water transport. Proximal tubular reabsorption in Necturus kidney. J Gen Physiol 66:671–733
Schiller A, Taugner R, Kriz W (1980a) The thin limbs of Henle's loop in the rabbit. A freeze fracture study. Cell Tissue Res 207:249–265
Schiller A, Forssmann WG, Taugner R (1980b) The tight junctions of the renal tubules in the cortex and outer medulla. A quantitative study of the kidneys of six species. Cell Tissue Res 212:395–413
Stetson DL, Wade JB, Giebisch G (1980) Morphologic alterations in the rat medullary collecting duct following potassium depletion. Kidney Int 17:45–56
Suzuki F, Nagano T (1978) Development of tight junctions in the caput epididymal epithelium of the mouse. Dev Biol 63:321–334
Taugner R, Boll U, Zahn P, Forssmann WG (1976) Cell junctions in the epithelium of Bowman's capsule. Cell Tissue Res 172:431–446
Tiedemann K (1979) Architecture of the mesonephric nephron in pig and rabbit. Anat Embryol 157:105–112
Tiedemann K, Wettstein R (1980) The mature mesonephric nephron of the rabbit embroy. I. SEM-studies. Cell Tissue Res 209:95–109
Ullrich KJ, Frömter E, Murer H (1979) Prinzipien des epithelialen Transportes in Niere und Darm. Klin Wochenschr 57:977–991
nVan Deurs B, Koehler JK (1979) Tight junctions in the choroid plexus epithelium. J Cell Biol 80:662–673
Wettstein R, Tiedemann K (1981) The mature mesonephric nephron of the rabbit embryo. II. TEM-studies. Cell Tissue Res 218:161–180
Wright EM, Diamond JM (1968) Effects of pH and polyvalent cations on the selective permeability of gallbladder epithelium to monovalent ions. Biochim Biophys Acta 163:57–74
Wright FS (1971) Increasing magnitude of electrical potential along the renal distal tubule. Am J Physiol 220:624–638
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schiller, A., Tiedemann, K. The mature mesonephric nephron of the rabbit embryo. Cell Tissue Res. 221, 431–442 (1981). https://doi.org/10.1007/BF00216746
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216746