Summary
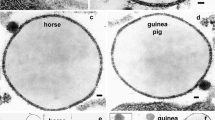
The plant lectin, concanavalin A (Con A) suppresses milk secretion when infused into the mammary gland or when incubated with lactating tissue in vitro. Toward defining its mode of action, we infused Con A into rat and goat mammary glands via the teats and observed effects on lactating cells. Lectin dosages were 2 and 25 mg per gland for rats and goat, respectively. Tissue samples were taken 1 and 3 h post infusion for rats and at 24 h for the goat. Control and Con A-treated tissues were observed by light microscopy and by both thin section and freeze fracture electron microscopy. In comparison to controls, Con A-treated tissues of both species exhibited alveoli with enlarged cells and relatively empty lumina; cells were distended with secretory vesicles and fat droplets. Apical plasma membranes of lectin-affected cells of the rat displayed a marked reduction in the number of microvilli, and exhibited an atypical branching and folded structure. Morphometry was employed to quantitate changes in cell and secretory product parameters in both rat and goat tissue. Microtubule numbers and distribution did not appear to be altered by Con A but considerable changes were noted in the arrangement of microfilaments associated with the secretory surface of lectin-treated epithelial cells. Various related ultrastructural changes and the role of Con A in perturbing the microfilament system are discussed.
Similar content being viewed by others
References
Amato PA, Loizzi RF (1979) The effects of cytochalasin B on glucose transport and lactose synthesis in lactating guinea pig mammary gland slices. Eur J Cell Biol 20:150–155
Amato PA, Loizzi RF (1981) The identification and localization of actin and actin-like filaments and lactating guinea pig mammary gland alveolar cells. Cell Motility 1:329–347
Buchheim W, Welsch U (1973) Evidence for the submicellar composition of casein micelles on the basis of electron microscopical studies. Neth Milk Dairy J 27:163–180
Burgess DR, Prum BE (1982) Re-evaluation of brush border motility: Calcium induces core filament solation and microvillar vesiculation. J Cell Bio1 94:97–107
Carron CP, Longo FJ (1982) Relation of cytoplasmic alkalinization to microvillar elongation and microfilament formation in the sea urchin egg. Dev Biol 89:128–137
Condeelis J (1979) Isolation of concanavalin A caps during various stages of formation and their association with actin and myosin. J Cell Biol 80:751–758
Craig SW, Pollard TS (1982) Actin-binding proteins. Trends Biochem Sci 7:88–92
Franke WW, Luder MR, Kartenbeck J, Zerban H, Keenan TW (1976) Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol 69:173–195
Gerson DG, Kiefer H, Eufe W (1982) Intracellular pH of mitogen-stimulated lymphocytes. Science 216:1009–1010
Keenan TW, Franke WW, Kartenbeck J (1974) Concanavalin A binding by solated plasma membranes and endomembranes from liver and mammary gland. FEBS Lett 44:274–278
Keenan TW, Franke WW, Mather IH, Morré DJ (1978) Endomembrane composition und function in milk formation. In: Lactation Vol 4. Academic Press, New York and London, pp 405–436
Knudson CM, Stemberger BH, Patton S (1978) Effects of colchicine on ultrastructure of the lactating mammary cell: Membrane involvement and stress on the Golgi apparatus. Cell Tissue Res 195:169–181
Lis H, Sharon N (1973) The biochemistry of plant lectins (phytohemagglutinins). A Rev Biochem 42:541–574
Murray LR, Powell KM, Sasaki M, Eigel WN, Keenan TW (1979) Comparison of lectin receptors and membrane coat-associated glycoproteins of milk lipid globule membranes. Comp Biochem Physiol 638:137–145
Nickerson SC, Keenan TW (1979) Distribution and orientation of microtubules in milk secreting epithelial cells of rat mammary gland. Cell Tissue Res 202:303–312
Nicolson GL (1974) The interaction of lectins with animal cell surfaces. Int Rev Cytol 39:90–178
Painter RG, Ginsburg M, Jaques B (1982) Concanavalin A induces interactions between surface glycoproteins and the platelet cytoskeleton. J Cell Bio1 92:565–573
Patton S (1976) Reversible suppression of milk secretion by concanavalin A. FEBS Lett 71:154–156
Patton S (1978) Milk secretion at the cellular level: a unique approach to the mechanism of exocytosis. J Dairy Sci 61:643–650
Patton S, Keenan TW (1975) The milk fat globule membrane. Biochim Biophys Acta 415:273–309
Patton S, Stemberger BH, Knudson CM (1977) The suppression of milk fat globule secretion by colchicine: An effect coupled to inhibition of exocytosis. Biochim Biophys Acta 499:404–410
Patton S, Bogus ER, Stemberger BH, Trams EG (1980a) Antiserum to the milk fat globule membrane: Preparation and capacity to suppress milk secretion. Biochim Biophys Acta 597:216–223
Patton S, Stemberger BH, Horton A, McCarl RL (1980b) Suppression of milk secretion (exocytosis) by concanavalin A in vitro. Biochim Biophys Acta 630:530–536
Roth J (1978) Compensatory membrane biogenesis and exocytosis as a result of Concanavalin A-induced membrane internalization. Exp Cell Res 114:31–38
Saacke RG, Heald CW (1974) Cytological aspects of milk formation and secretion. In: Lactation Vol 2. Academic Press, New York and London, pp 148–189
Sasaki M, Keenan TW (1979) Ultrastructural characterization of carbohydrate distribution on milk lipid globule membrane. Cell Biol Int Rep 3:67–74
Singh S, White FC, Bloor CM (1981) Myocardial morphometric characteristics in swine. Circ Res 49:434–441
Smith JJ, Nickerson SC, Keenan TW (1982) Metabolic energy and cytoskeletal requirements for synthesis and secretion by acini from rat mammary gland — I. Ultrastructural and biochemical aspects of synthesis and release of milk proteins. Int J Biochem 14:87–98
Toyashima S, Iwata M, Osawa T (1976) Kinetics of lymphocyte stimulation by concanavalin A. Nature 264:447–449
Welsch U, Singh S, Buchheim W, Patton S (1982) Evidence of plasma membrane recycling in the lactating cell by concanavalin A-ferritin labeling. J. Dairy Sci 65, Suppl. 1:83–84
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welsch, U., Singh, S., Stemberger, B.H. et al. Ultrastructural changes in lactating tissue related to the suppression of milk secretion by concanavalin A. Cell Tissue Res. 230, 527–541 (1983). https://doi.org/10.1007/BF00216199
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216199