Summary
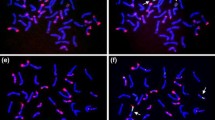
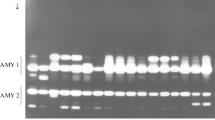
Genetic mapping of polymorphic C-bands allows direct comparisons between genetic and physical maps. Eleven C-bands and two seed storage protein genes on chromosome 1B, polymorphic between Langdon durum and four accessions of T. dicoccoides, were used to study the distribution of recombination along the entire length of the chromosome. Recombination in the short arm was almost completely restricted to the satellite, two-thirds of the arm's length from the centromere; the Gli-B1 gene was found to be tightly linked to the telomeric C-band. In the long arm, the distal 51.4% of the arm accounted for 88% of recombination; the proximal half of the arm accounted for the remaining 12%. While the amount of crossing-over differed significantly between the four T. dicoccoides 1B chromosomes, there were no significant differences in the relative distributions of crossing-over along the chromosome. Consequently, the genetic maps obtained from the four individual T. dicoccoides chromosomes were combined to yield a consensus map of 14 markers (including the centromere) for the chromosome.
Similar content being viewed by others
References
Appels R, Gerlach WL, Dennis ES, Swift H, Peacock WJ (1980) Molecular and chromosomal organization of DNA seding for the ribosomal RNAs in cereals. Chromosoma 78: 293–311
Curtis C, Feldman M (1988) Increased proximal recombination frequency in common wheat by premeiotic colchicine treatment. Proc 7th Int Wheat Genet Symp, Cambridge, pp 243–248
Cusick JE, McIntosh RA (1987) Linkage map of wheat (Triticum aestivum: 2n=42). In: O'Brien SJ (ed) Genetic maps 1987, vol 4. Cold Spring Harbor Laboratory Press, Cold Spring Harbor/NY, pp 670–677
Dvorak J, Appels R (1986) Investigation of homologous crossing-over and sister chromatid exchange in the wheat NorB2 locus coding for rRNA and the GliB2 locus coding for gliadins. Genetics 113: 1037–1056
Dvorak J, Chen K-C (1984) Distribution of nonstructural variation between wheat cultivars along chromosome 6Bp: evidence from the linkage map and the physical map of the arm. Genetics 106: 325–333
Dvorak J, Chen K-C, Giorgi B (1984) The C-band pattern of a Ph mutant of durum wheat. Can J Gen Cytol 26: 360–363
Dvorak J, McGuire PE (1981) Nonstructural chromosome differentiation among wheat cultivars, with special reference to differentiation of chromosomes in related species. Genetics 97: 391–414
Endo TR, Mukai Y (1988) Chromosome mapping of a speltoid suppression gene of Triticum aestivum L. based on partial deletions in the long arm of chromosome 5A Jpn J Gen 63: 501–505
Fu TK, Sears ER (1973) The relationship between chiasmata and crossing-over in Triticum aestivum. Genetics 75: 231–246
Galili G, Feldman M (1983a) Genetic control of endosperm proteins in wheat. 1. The use of high resolution, one-dimensional gel electrophoresis for the allocation of genes coding for endosperm protein subunits in the common wheat cultivar ‘Chinese Spring.’ Theor Appl Genet 64: 97–101
Galili G, Feldman M (1983b) Genetic control of endosperm proteins in wheat. 2. Variation in high-molecular-weight glutenin and gliadin subunits of Triticum aestivum. Theor Appl Genet 66: 77–86
Galili G, Feldman M (1984) Mapping of glutenin and gliadin genes located on chromosome 1B of common wheat. Mol Gen Genet 193: 293–298
Gillies CB, Lukaszewski AJ (1989) Synaptonemal complex formation in rye (Secale cereale) heterozygous for telomeric C-bands. Genome 32: 901–907
Hart GE, Gale MD (1987) Biochemical/molecular loci of hexaploid wheat. In: O'Brien SJ (ed) Genetic maps 1987, vol 4. Cold Spring Harbor Laboratory Press, Cold Spring Harbor/NY, pp 678–684
Hutchinson J, Lonsdale DM (1982) The chromosomal distribution of cloned highly repetitive sequences from hexaploid wheat. Heredity 48: 371–376
Jampates R, Dvorak J (1986) Location of the Ph1 locus in the metaphase chromosome map and the linkage map of the 5Bq arm of wheat. Can J Genet Cytol 28: 511–519
Joppa LR (1988) Cytogenetics of tetraploid wheat. Proc 7th Int Wheat Genet Symp, Cambridge, pp 197–202
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12: 172–175
Kota RS, Dvorak J (1986) Mapping of a chromosome pairing gene and 5S rRNA genes in Triticum aestivum L.tby a spontaneous deletion in chromosome arm 5Bp. Can J Genet Cytol 28: 266–271
Lawrence GJ, Appels R (1986) Mapping the nucleolus organizer region, seed protein loci, and isozyme loci on chromosome 1R in rye. Theor Appl Genet 71: 742–749
Linde-Larsen I (1979) Giemsa C-banding of barley chromosomes. III. Segregation and linkage of C-bands on chromosomes 3, 6, and 7. Hereditas 91: 73–77
Lukaszewski AJ, Gustafson JP (1983) Translocations and modifications of chromosomes in Triticale x wheat hybrids. Theor Appl Genet 64: 239–248
Miller TE, Gerlach WL, Flavell RB (1980) Nucleolus organizer variation in wheat and rye revealed by in situ hybridization. Heredity 45: 377–382
Mukai Y, Endo TR, Gill BS (1990) Physical mapping of 5S rRNA multigene family in common wheat. J Heredity 81: 290–295
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu Rev Plant Physiol 38: 141–153
Payne PI, Holt LM, Worland AJ, Law CN (1982) Structural and genetical studies on the high-molecular-weight subunits of wheat glutenin. 3. Telocentric mapping of the subunit genes on the long arms of the homoeologous group 1 chromosomes. Theor Appl Genet 63: 129–138
Payne PI, Holt LM, Hutchinson J, Bennett MD (1984) Development and characterization of a line of bread wheat, Triticum aestivum, which lacks the short-arm satellite of chromosome 1B and the GliB1 locus. Theor Appl Genet 68: 327–334
Rayburn AL, Gill BS (1986) Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol Biol Rep 4: 102–109
Sears ER (1984) Mutations in wheat that raise the level of meiotic chromosome pairing. In: Gustafson JP (ed) Gene manipulation in plant improvement, 16th Stadler Genetics Symposium. Plenum Press, New York, pp 295–300
Singh NK, Shepherd KW (1988a) Linkage mapping of genes controlling endosperm storage proteins in wheat. 1. Genes on the short arms of group 1 chromosomes. Theor Appl Genet 75: 628–641
Singh NK, Shepherd KW (1988b) Linkage mapping of genes controlling endosperm storage proteins in wheat. 2. Genes on the long arms of group 1 chromosomes. Theor Appl Genet 75: 642–650
Snape JW, Flavell RB, O'Dell M, Hughes WG, Payne PI (1985) Intrachromosomal mapping of the nucleolar organizer region relative to three marker loci on chromosome 1B of wheat (Triticum aestivum). Theor Appl Genet 69: 263–270
Author information
Authors and Affiliations
Additional information
Communicated by K. Tsunewaki
Rights and permissions
About this article
Cite this article
Curtis, C.A., Lukaszewski, A.J. Genetic linkage between C-bands and storage protein genes in chromosome 1B of tetraploid wheat. Theoret. Appl. Genetics 81, 245–252 (1991). https://doi.org/10.1007/BF00215730
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00215730