Summary
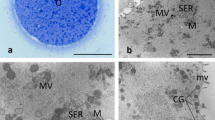
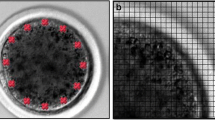
During meiotic maturation, the cortex of oocytes of Xenopus laevis undergoes structural reorganization, visualized in this study by freeze-fracture electron microscopy. In the full-grown but immature oocyte, annulate lamellae are dispersed throughout the subcortex of the egg, 5 to 20 μm from the plasma membrane. The annulate lamellae consist of well-organized stacks of membrane with visible pores. Stimulation of meiotic maturation by progesterone leads to disruption of the annulate lamellae and formation of an elaborate cortical endoplasmic reticulum which surrounds the cortical granules and intertwines throughout the cortex of the mature egg. Pore-like structures similar to those previously observed in the subcortical annulate lamellae are observed in the mature cortical endoplasmic reticulum. The cortical endoplasmic reticulum is often in close apposition with the plasma membrane and with membranes of cortical granules, but no junctions are visualized. This study provides further evidence that the cortical endoplasmic reticulum develops during progesterone-stimulated meiotic maturation in vitro, and that the annulate lamellae are precursors to the cortical endoplasmic reticulum.
Similar content being viewed by others
References
Balinsky BI (1966) Changes in the ultrastructure of amphibian eggs following fertilization. Acta Morphol Embryol Exp 9:132–154
Balinsky BI, Devis RJ (1963) Origin and differentiation of cytoplasmic structures in the oocytes of Xenopus laevis. Acta Morphol Embryol Exp 6:55–108
Bluemink JG, Hage WJ, van den Hoef MHF, Dictus WJAG (1983) Freeze-fracture electron microscopy of membrane changes in progesterone-induced maturing oocytes and eggs of Xenopus laevis. Eur J Cell Biol 31:85–93
Busa WB, Nuccitelli R (1985) An elevated free cytosolic Ca2+ wave follows fertilization in eggs of the frog, Xenopus laevis. J Cell Biol 100:1325–1329
Campanella C, Andreuccetti P (1977) Ultrastructural observations on cortical endoplasmic reticulum and on residual cortical granules in the egg of Xenopus laevis. Dev Biol 56:1–10
Campanella C, Andreuccetti P, Taddei C, Talevi R (1984) The modifications of cortical endoplasmic reticulum during in vitro maturation of Xenopus laevis oocytes and its involvement in cortical granule exocytosis. J Exp Zool 229:283–293
Chambers EL, Pressman BC, Rose B (1974) The activation of sea urchin eggs by the divalent ionophores A23187 and X-537A. Biochem Biophys Res Commun 60:126–132
Charbonneau M, Grey RD (1984) The onset of activation responsiveness during maturation coincides with the formation of the cortical endoplasmic reticulum in oocytes of Xenopus laevis. Dev Biol 102:90–97
Eisen A, Kiehart DP, Wieland SJ, Reynolds GT (1984) Temporal sequence and spatial distribution of early events of fertilization in single sea urchin eggs. J Cell Biol 99:1647–1654
Elinson RP (1980) The amphibian egg cortex in fertilization and early development. In: Subtelny S, Wessels NK (eds) The cell surface: Mediator of developmental processes. Academic Press, New York, pp 217–234
Epel D (1980) Experimental analysis of the role of intracellular calcium in the activation of the sea urchin egg at fertilization. In: Subtelny S, Wessels NK (eds) The Cell Surface: Mediator of Developmental Processes. Academic Press, New York, pp 169–183
Gardiner DM, Grey RD (1983) Membrane junctions in Xenopus eggs: Their distribution suggests a role in calcium regulation. J Cell Biol 96:1159–1163
Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT (1978) A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol 76:448–466
Grey RD, Wolf DP, Hedrick JL (1974) Formation and structure of the fertilization envelope in Xenopus laevis. Dev Biol 36:44–61
Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L (1979) Synapic vesicle exocytosis captured by quick-freezing and correlated with guantal transmitter release. J Cell Biol 81:275–300
Imoh H, Okamoto M, Eguchi G (1983) Accumulation of annulate lamellae in the subcortical layer during progesterone-induced oocyte maturation in Xenopus laevis. Dev Growth Differ 25(1):1–10
Kemp NE, Istock NL (1967) Cortical changes in growing oocytes and in fertilized or pricked eggs of Rana pipiens. J Cell Biol 34:111–122
Kessel RG (1968) Annulate lamellae. J Ultrastr Res [Suppl] 10:1–82
Kessel RG, Subtelny S (1981) Alteration of annulate lamellae in the in vitro progesterone-treated, full-grown Rana pipiens oocyte. J Exp Zool 217:119–135
Kubota HY, Yoshimoto Y, Yoneda M, Hiramoto Y (1987) Free calcium wave upon activation in Xenopus eggs. Dev Biol 119:129–136
Masui Y, Clarke HJ (1979) Oocyte maturation. Rev Cytology 57:185–282
Schmidt T, Patton C, Epel D (1982) Is there a role for Ca2+ influx during fertilization of the sea urchin egg? Dev Biol 90:284–290
Shapiro B, Eddy EM (1980) When sperm meets egg: Biochemical mechanisms of gamete interaction. Int Rev Cytol 66:257–295
Stafstrom JP, Staehelin A (1984) Are annulate lamellae in the Drosophila embryo the result of overproduction of nuclear pore components? J Cell Biol 98:699–708
Steinhardt RA, Epel D, Carroll EJ, Yanagimachi R (1974) Is calcium ionophore a universal activator for unfertilized eggs? Nature (London) 252:41–43
Vacquier V (1981) Dynamic changes of the egg cortex. Dev Biol 84:1–26
Wasserman WJ, Houle JG, Samuel D (1984) The maturation response of stage IV, V, and VI Xenopus oocytes to progesterone stimulation in vitro. Dev Biol 105:315–324
Wasserman WJ, Penna MJ, Houle JG (1986) The regulation of Xenopus laevis oocyte maturation. In: Gall JG (ed) Gametogenesis and the Early Embryo. Alan R. Liss, Inc. New York, pp 111–130
Wolf DP (1974) The cortical response in Xenopus laevis ova. Dev Biol 40:102–115
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Larabell, C.A., Chandler, D.E. Freeze-fracture analysis of structural reorganization during meiotic maturation in oocytes of Xenopus laevis . Cell Tissue Res. 251, 129–136 (1988). https://doi.org/10.1007/BF00215457
Issue Date:
DOI: https://doi.org/10.1007/BF00215457