Summary
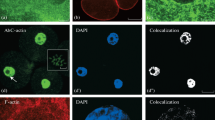
Cytoskeletons of primitive erythrocytes have been isolated from the embryos of day 12 pregnant C57/Bl mice and examined by transmission electron microscopy, immunofluorescence microscopy, and SDS-polyacrylamide gel electrophoresis. Microtubules are the most prominent cytoskeletal component. They are found either singly or organized into loose bundles just under the plasma membrane, but do not form classical marginal bands in most cells. Immunofluorescence with a polyclonal tubulin antiserum confirms this distribution and further reveals numerous mitotic figures among the cells. Rhodamine-conjugated phalloidin and heavy meromyosin labeling reveal that actin is localized in the cortex of the primitive erythrocyte in the form of 6 nm filaments. Antibody directed against avian erythrocyte alpha spectrin demonstrates that spectrin is also found in the cortex. Occasional 10-nm intermediate filaments, observed in the primitve erythrocytes by electron microscopy, are believed to be of the vimentin class based on positive reaction of the cells with vimentin-specific antiserum. In addition, a band in erythrocyte cytoskeletons comigrates in SDS-polyacrylamide gels with vimentin isolated from mouse kidney. Spectrin and actin were also found to be associated with the membrane of primitive erythrocytes when membrane ghost preparations were analyzed by SDS-polyacrylamide gel electrophoresis.
Similar content being viewed by others
References
Barret LA, Dawson RB (1974) Avian erythrocyte development: microtubules and the formation of the disk shape. Dev Biol 36:72–81
Behnke O (1970) A comparative study of microtubules of discshaped blood cells. J Ultrastruct Res 31:61–75
Branton D, Cohen M, Tyler J (1981) Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell 24:24–32
Cohen WD (1978) Observations on the marginal band of nucleated erythrocytes. J Cell Biol 78:260–273
Cohen WD, Bartelt D, Jaeger R, Langford G, Nemhauser I (1982) The cytoskeletal system of nucleated erythrocytes. I. Composition and function of major elements. J Cell Biol 93:828–838
Craig ML, Russell ES (1964) A developmental change in hemoglobins correlated with embryonic red cell population in the mouse. Dev Biol 10:191–201
Fawcett DW, Witebsky F (1964) Observations on the ultrastructure of nucleated erythrocytes and thrombocytes with particular reference to the structural basis of their discoidal shape. Z Zellforsch 62:785–806
Georgatos SD, Marchesi VT (1985) The binding of vimentin to human erythrocyte membranes: a model system for the study of intermediate filament-membrane interactions. J Cell Biol 100:1955–1961
Goniakowska-Witalinska L, Witalinski W (1976) Evidence for a correlation between the number of marginal band microtubules and the size of vertebrate erythrocytes. J Cell Sci 22:397–401
Granger BL, Repasky EA, Lazaridies E (1982) Synemin and vimentin are components of intermediate filaments in avian erythrocytes. J Cell Biol 92:299–312
Huxley H (1963) Protein filaments from muscle. J Mol Biol 7:281–308
Joseph-Silverstein J, Cohen WD (1984) The cytoskeletal system of nucleated erythrocytes. III. Marginal band function in mature cells. J Cell Biol 98:2118–2125
Joseph-Siverstein J, Cohen WD (1985) Role of the marginal band in an invertebrate erythrocytc: evidence for a universal mechanical function. Can J Biochem Cell Biol 63:621–630
Kovach JS, Marks PA, Russell ES, Epler H (1967) Erythroid cell development in fetal mice: ultrastructural characteristics and hemoglobin synthesis. J Mol Biol 25:131–142
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (Lond) 227:680–685
Meves F (1911) Gesammelte Studien an den roten Blutkörperchen der Amphibien. Arch Mikrosk Anat Entwicklungsmech 77:465–540
Murphy DB, Grasser WA, Wallis K (1986) Immunofluorescence examination of beta tubulin expression and marginal band formation in developing chicken erythroblasts. J Cell Biol 102:628–635
Nemhauser I, Ornberg R, Cohen WD (1980) Marginal bands in blood cells of invertebrates. J Ultrastruct Res 70:308–317
Repasky EA, Eckert BS (1981) Microtubules in mammalian erythroblasts. Are marginal bands present? Anat Embryol 162:419–424
Repasky EA, Granger BL, Lazaridies E (1982) Widespread occurence of avian spectrin in nonerythroid cells. Cell 29:821–833
Rodriguez J, Deinhardt F (1960) Preparation of a semi-permeable mounting medium for fluorescent antibody studies. Virology 12:316–317
Schliwa M, van Blerkom J (1981) Structural interaction of cytoskeletal components. J Cell Biol 90:222–235
Sommer JR (1977) To cationize glass. J Cell Biol 75:745a (Abstr.)
Spurr A (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Szent-Gyorgyi AG (1953) Meromyosin, the subunit of myosin. Arch Biochem Biophys 42:305–320
Venable J, Coggeshall R (1965) A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25:407–408
van Deurs B, Behnke O (1973) The microtubule marginal band of mammalian red blood cells. Z Anat Entwicklungsgesch 143:43–47
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koury, S.T., Repasky, E.A. & Eckert, B.S. The cytoskeleton of isolated murine primitive erythrocytes. Cell Tissue Res. 249, 69–77 (1987). https://doi.org/10.1007/BF00215420
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00215420