Summary
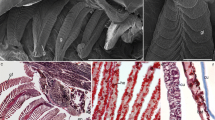
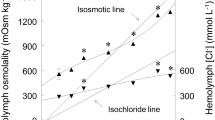
This study provides information on the rates of DNA synthesis, sites of DNA synthesis, and DNA content of the avian salt gland during the osmoticstressing (plasma membrane synthesis) and destressing (plasma membrane turnover) cycle, in an effort to better understand the relationship of cell turnover to the initial events in plasma membrane amplification, differentiation, and turnover. The rate of DNA synthesis increases 12–24 h after the onset of osmotic stress, is maximal at about 24 h of osmotic stress, and decreases thereafter in fully stressed and destressed glands. The maximum DNA and protein content, and the maximum protein/DNA ratio are obtained after about 3 days of stress. Autoradiograms show that at 24 h of stress 70–80% of DNA synthesis occurs in connective tissue cells and 20–30% in parenchymal cells, but by 6 days of stress, synthesis occurs about equally in these cell groups. Because destressing is characterized by a large decrease in plasma membrane and in glandular protein, but by little DNA turnover or loss, the loss of plasma membrane is likely due to some type of cell dedifferentiation rather than cell turnover.
Similar content being viewed by others
References
Brunk CF, Jones KC, James TW (1979) Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem 92:497–500
Croft DN, Lubran M (1965) The estimation of deoxyuribonucleic acid in the presence of sialic acid: Application to analysis of human gastric washings. Biochem J 95:612–620
Ellis RA, Goertemiller CC Jr, DeLellis RA, Kablotsky YH (1963) The effect of a salt water regimen on the development of the salt glands of domestic ducklings. Dev Biol 8:286–308
Ernst SA (1972) Transport adenosine triphosphatase cytochemistry. II. Cytochemical localization of ouabain-sensitive, potassium-dependent phosphatase activity in the secretory epithelium of the avian salt gland. J Histochem Cytochem 20:23–28
Ernst SA, Ellis RA (1969) The development of surface specialization in the secretory epithelium of the avian salt gland in response to osmotic stress. J Cell Biol 40:305–321
Holmes WN, Stewart DJ (1968) Changes in nucleic acid and protein composition of the nasal glands from the duck (Anas platyrhynchos) during the period of adaptation to hypertonic saline. J Exp Biol 48:509–519
Hossler FE, Sarras MP Jr, Allen ER (1978a) Ultrastructural, cyto-and biochemical observations during turnover of plasma membrane in duck salt gland. Cell Tissue Res 188:299–315
Hossler FE, Sarras MP Jr, Barrnett RJ (1978b) Ouabain binding during plasma membrane biogenesis in duck salt gland. J Cell Sci 31:179–197
Levine AM, Higgins JA, Barrnett RJ (1972) Biogenesis of plasma membranes in salt glands of salt stressed domestic ducklings: Localization of acyltransferase activity. J Cell Sci 11:855–873
Lingham RB, Stewart DJ, Sen AK (1980) The induction of (NA++K+)-ATPase in the salt gland of the duck. Biochim Biophys Acta 601:229–234
Lowry OH, Rosebrough WF, Farr AL, Randall RV (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mazurkiewicz JE, Barrnett RJ (1981) Organotypic cultures of the avian salt gland: biosynthesis of membrane proteins. J Cell Sci 48:75–88
Mollenhauer HH (1964) Plastic embedding mixtures for use in electron microscopy. Stain Technol 39:111–114
Palade GE (1959) Functional changes in structure of cell components. In: Hayashi T (ed) Subcellular particles. Ronald Press, New York, pp 64–83
Peaker M, Linzell JL (1975) Salt glands in reptiles and birds. Cambridge Press, London
Pollack LR, Tate EH, Cook JS (1981) Turnover and regulation of Na-K-ATPase in HeLa cells. Am J Physiol 241:C173-C183
Schimke RT (1975) Turnover of membrane proteins in animal cells. Meth Membr Biol 3:201–236
Schmidt-Nielsen K (1960) The salt-secreting gland of marine birds. Circulation 21:955–967
Stewart DJ, Semple EW, Swart GT, Sen AK (1976) Induction of the catalytic protein of (Na+ + K+)-ATPase in the salt gland of the duck. Biochim Biophys Acta 419:150–163
Tweto J, Doyle D (1977) Turnover of proteins of the eukaryotic cell surface. In: Poste G, Nicolson GL (eds) The Synthesis, assembly and turnover of cell surface components. North Holland Publishing Co, New York, pp 137–163
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hossler, F.E. On the mechanism of plasma membrane turnover in the salt gland of ducklings. Cell Tissue Res. 226, 531–540 (1982). https://doi.org/10.1007/BF00214782
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214782