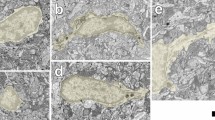
Abstract
Cell death is frequent during the development of the nervous system. In the developing optic nerve of chicks and quails, neuroepithelial cell death was first observable on the third day of incubation, slightly after the first cell ganglion axons appeared in the stalk. Specialized phagocytes were observed within the stalk in chronological and topographical coincidence with cell death. These cells were identified as macrophages because of their morphological features, intense acid phosphatase activity and, in quail embryos, labeling with QH1, a monoclonal antibody recognizing quail hemangioblastic cells. Macrophages in areas of cell death were round and actively phagocytosed cell debris. We used electron microscopy and histochemical and immunocytochemical labeling to study macrophagic cells of the optic nerve in avian embryos of 3–6.5 days of incubation. As development proceeded, phagocytosing, round macrophages became ameboid macrophages that migrated from areas of cell death toward regions occupied by optic axonal fascicles. Macrophages in these locations were thin and elongated, with a few processes. To elucidate the final fate of macrophagic cells in the optic nerve, sections taken from older embryonic and hatched quails were stained with the QH1 antibody. On the 8th day of incubation some slightly ramified QH1+ cells were present among axonal fascicles. In subsequent stages these cells increased in number and acquired more complex ramifications. In adult optic nerves, QH1+ cells had a small body and sent out slender processes, sometimes with secondary and tertiary branches, which were frequently orientated parallel to the course of the optic axons. These cells were considered to be microglial cells. The appearance of macrophages within the developing optic nerve at the same time as neuroepithelial cell death suggests that cell death influences the recruitment of macrophages into the nerve. When macrophages reach the areas invaded by optic axonal fascicles, they undergo structural and probably also physiological changes that appear to signal differentiation into microglia.
Similar content being viewed by others
References
Ashwell, K (1991) The distribution of microglia and cell death in the fetal rat forebrain. Brain Res Dev Brain Res 58:1–12
Bonetti B, Monaco S, Giannini C, Ferrari S, Zanusso GL, Rizzuto N (1993) Human peripheral nerve macrophages in normal and pathological conditions. J Neurol Sci 118:158–168
Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R (1991) Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron 6:359–370
Clarke PGH (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Colello RJ (1987) The relationship of axonal growth cones to macrophage-like cells in the eye stalk of the embryonic mouse. J Anat 152:264
Cuadros MA, Ríos A (1988) Spatial and temporal correlation between early nerve fiber growth and neuroepithelial cell death in the chick embryo retina. Anat Embryol 178:543–551
Cuadros MA, García-Martín M, Martin C, Ríos A (1991) Haemopoietic phagocytes in the early differentiating avian retina. J Anat 177:145–158
Cuadros MA, Coltey P, Nieto MC, Martin C (1992a) Demonstration of a phagocytic cell system belonging to the hemopoietic lineage and originating from the yolk sac in the early avian embryo. Development 115:157–168
Cuadros MA, Moujahid A, Martín-Partido G, Navascués J (1992b) Microglia in the mature and developing quail brain as revealed by a monoclonal antibody recognizing hemopoietic cells. Neurosci Lett 148:11–14
Cuadros MA, Martin C, Coltey P, Almendros A, Navascués J (1993) First appearance, distribution, and origin of macrophages in the early development of the avian central nervous system. J Comp Neurol 330:113–129
Cuadros MA, Moujahid A, Quesada A, Navascués J (1994) Development of microglia in the quail optic tectum. J Comp Neurol 348:207–224
David S, Bouchard C, Tsatas O, Giftochristos N (1990) Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron 5:463–469
Davis EJ, Foster TD, Thomas WE (1994) Cellular forms and functions of brain microglia. Brain Res Bull 34:73–78
Dowding AJ, Maggs A, Scholes J (1991) Diversity amongst the microglia in growing and regenerating fish CNS: immunohistochemical characterization using FL.1, an anti-macrophage monoclonal antibody. Glia 4:345–364
Goodbrand IA, Gaze R (1991) Microglia in tadpoles of Xenopus laevis: normal distribution and the response to optic nerve injury. Anat Embryol 184:71–82
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Higuchi S, Suga M, Dannenberg AM, Schofield BH (1979) Histochemical demonstration of enzyme activities in plastic and paraffin embedded tissue sections. Stain Technol 54:5–12
Homma S, Yaginuma H, Oppenheim RW (1994) Programmed cell death during the earliest stages of spinal cord development in the chick embryo: a possible means of early phenotypic selection. J Comp Neurol 345:377–395
Horsburgh GM, Sefton AJ (1986) The early development of the optic nerve and chiasm in embryonic rat. J Comp Neurol 243:547–560
Hume DA, Perry VH, Gordon S (1983) Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol 97:253–257
Lawson LJ, Frost L, Risbridger J, Fearn S, Perry VH (1994) Quantification of the mononuclear phagocyte response to Wallerian degeneration of the optic nerve. J Neurocytol 23:729–744
Ling EA, Wong WC (1993) The origin and nature of ramified and ameboid microglia: a historical review and current concepts. Glia 7:9–18
Ludwin SK (1990) Phagocytosis in the rat optic nerve following Wallerian degeneration. Acta Neuropathol 80:266–273
Martín-Partido G, Navascués J (1990) Macrophage-like cells in the presumptive optic pathways in the floor of the diencephalon of the chick embryo. J Neurocytol 19:820–832
Martín-Partido G, Cuadros MA, Martin C, Coltey P, Navascués J (1991) Macrophage-like cells invading the suboptic necrotic centres of the avian embryo diencephalon originate from haemopoietic precursors. J Neurocytol 20:962–968
Milligan CE, Levitt P, Cunningham TJ (1991) Brain macrophages and microglia respond differently to lesions of the developing and adult visual system. J Comp Neurol 314:136–146
Naujoks-Manteuffel C, Niemann U (1994) Microglial cells in the brain of Pleurodeles waltl (Urodela, Salamandridae) after Wallerian degeneration in the primary visual system using Bandeiraea simplicifolia isolectin B4-cytochemistry. Glia 10:101–113
Navascués J, Rodríguez-Gallardo L, Martín-Partido G, Alvarez IS (1985) Proliferation of glial precursors during the early development of the chick embryo optic nerve. Anat Embryol 172:365–373
Navascués J, Martín-Partido G, Alvarez IS, Rodríguez-Gallardo L (1988) Cell death in suboptic necrotic centers of chick embryo diencephalon and their topographic relationship with the earliest optic fiber fascicles. J Comp Neurol 278:34–46
Navascués J, González-Ramos C, Alvarez IS, Rodríguez-Gallardo L, Martín-Partido G (1989) Quantitative studies of mitotic cells in the chick embryo optic stalk during the early period of invasion by optic fibres. Anat Embryol 180:343–351
Navascués J, Moujahid A, Quesada A, Cuadros MA (1994) Microglia in the avian retina: immunocytochemical demonstration in the adult quail. J Comp Neurol 350:171–186
Navascués J, Moujahid A, Almendros A, Marín-Teva JL, Cuadros MA (1995) Origin of microglia in the quail retina: central-to-peripheral and vitreal-to-scleral migration of microglial precursors during development. J Comp Neurol 354:209–228
Oppenheim RW (1991) Cell death during development of the nervous system. Annu Rev Neurosci 14:453–501
Pardanaud L, Altmann C, Kitos P, Dieterlen-Lièvre F, Buck CA (1987) Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100:339–349
Perry VH, Gordon S (1988) Macrophages and microglia in the nervous system. Trends Neurosci 11:273–277
Perry VH, Hume DA, Gordon S (1985) Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15:313–326
Peters A, Palay SL, Webster HF (1976) The fine structure of the nervous system: the neurons and supporting cells. Saunders, Philadelphia
Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD (1993) Programmed cell death and the control of cell survival: lessons from the nervous system. Science 262:695–700
Rager G (1980) Development of the retinotectal projection in the chicken. Adv Anat Embryol Cell Biol 63:1–93
Rager G (1983) Structural analysis of fiber organization during development. In: Changeux JP, Glowinski J, Imbert M, Bloom FE (eds) Progress in brain research, vol 58. Molecular and cellular interactions underlying higher brain functions. Elsevier, Amsterdam, pp 313–319
Rio-Hortega P (1932) Microglia. In: Penfield W (ed) Cytology and cellular pathology of the nervous system, vol 2. Hoeber, New York, pp 483–534
Silver J, Hughes AFW (1973) The role of cell death during morphogenesis of the mammalian eye. J Morphol 140:159–170
Silver J, Sidman RL (1980) A mechanism for the guidance and topographic patterning of retinal ganglion cell axons. J Comp Neuroll 189:101–111
Silver J, Poston M, Rutishauser U (1987) Axon pathway boundaries in the developing brain. I. Cellular and molecular determinants that separate the optic and olfactory projections. J Neurosci 7:2264–2272
Stoll G, Trapp BD, Griffin JW (1989) Macrophage function during Wallerian degeneration of rat optic nerve: clearance of degenerating myelin and Ia expression. J Neurosci 9:2327–2335
Stolz B, Erulkar S, Kuffler DP (1991) Macrophages direct process elongation from adult frog motorneurons in culture. Proc R Soc Lond [Biol] 244:227–231
Strongin AC, Guillery RW (1981) The distribution of melanin in the developing optic cup and stalk and its relation to cellular degeneration. J Neurosci 1:1193–1204
Sturrock RR (1984) Microglia in the human embryonic optic nerve. J Anat 139:81–91
Turner JE (1975) Non-glial phagocytes within the degenerating optic nerve of the newt (Triturus viridiscens). J Exp Zool 193:87–100
Ulshafer RJ, Clavert A (1979) Cell death and optic fiber penetration in the optic stalk of the chick. J Morphol 162:67–76
Vaughn JE, Peters A (1968) A third neuroglial cell type. An electron microscopic study. J Comp Neurol 133:269–288
Wilson MA, Gaze R, Goodbrand IA, Taylor JSH (1992) Regeneration in the Xenopus tadpole is preceded by a massive macrophage/microglial response. Anat Embryol 186:75–89
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moujahid, A., Navascués, J., Marín-Teva, J.L. et al. Macrophages during avian optic nerve development: relationship to cell death and differentiation into microglia. Anat Embryol 193, 131–144 (1996). https://doi.org/10.1007/BF00214704
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214704