Summary
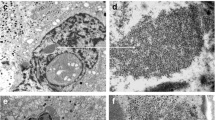
The synthetic pathways of proteins and catecholamines in the rat adrenal medullary cells were compared systematically at the ultrastructural level, within a 24 h period, with 2 tracers, L-tyrosine 3,5-3H and L-3,4-dihydroxy [ring 2,5,6-3H] phenylalanine (L-dopa3H). Young rats were injected with either of these tracers and sacrificed in pairs at close time intervals. With L-tyrosine 3H, the label was about equal over rough endoplasmic reticulum (RER) and secretory granules at 2 min after injection and remained almost constant in intensity over the secretory granules throughout the period of observation. A peak of radioactivity was also observed in the Golgi complex between 5 and 20 min after injection. This indicates that L-tyrosine 3H participates in the synthesis of both granule proteins and catecholamines as confirmed by the results obtained after injection of L-dopa 3H. With this tracer, radioactivity over RER, Golgi complex, cytosol and cell surface remained very low at all times and was undetectable at several time intervals. In contrast, radioactivity over secretory granules was very high at all time intervals. The present results thus confirm that in both adrenaline- and noradrenaline-storing cells, the protein moiety of chromaffin granules is synthetized in the RER, packaged in the Golgi complex and rapidly found in newly formed secretory granules. Following either L-tyrosine 3H or L-dopa 3H injection, catecholamine synthesis occurs only in or in close vicinity to chromaffin granules in both cell types at all time intervals.
Similar content being viewed by others
References
Benchimol S, Cantin M (1978) Ultrastructural radioautography of the incorporation of tritiated leucine by the rat adrenal medulla in vivo. Cell Tissue Res 193:179–199
Blaschko H, Hagen P, Welch AD (1955) Observations on the intracellular granules of the adrenal medulla. J Physiol 129:27–49
Burri PH, Giger H, Gnadi HR, Weibel ER (1968) Application of stereological methods to cytophysiologic experiments in polarized cells. Proceedings of the Fourth European Conference on Electron Microscopy, Rome 1:593–594
Cantin M, Benchimol S (1975) Localization and characterization of carbohydrates in adrenal medullary cells. J Cell Biol 65:463–479
Cantin M, Araujo-Nascimento M de F, Benchimol S, Désormeaux Y (1977) Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries and arterioles of the ischemic (endocrine) kidney. Am J Physiol 87:581–602
Claude A (1970) Growth and differentiation of cytoplasmic membranes in the course of lipoprotein granule synthesis in the hepatic cell. I. Elaboration of the elements of the Golgi complex. J Cell Biol 47:745–766
Coupland RE, Kobayashi S (1976) Recent studies on the fixation of adrenaline and storage in chromaffin cells. In: Coupland RE, Fujita T (eds) Chromaffin, entero-chromaffm and related cells. Elsevier Scientific Publishing Co
Coupland RE, Kobayashi S, Crowe J (1976a) On the fixation of catecholamines including adrenaline in tissue sections. J Anat 122:403–413
Coupland RE, Kobayashi S, Kent C (1976b) Observations on the localization of recently synthetized catecholamines in chromaffin cells after the injection of L-2–5, 6-3H DOPA. J Endocrinol 69:139–148
DaPrada M, Berlepsch K von, Pletscher A (1972) Storage of biogenic amines in blood platelets and adrenal medulla. Naunyn-Schmiedebergs Arch Pharmakol 275:315–322
Eade NR (1958) The distribution of the catecholamines in homogenates of the bovine adrenal medulla. J Physiol 141:183–192
Elfvin LC, Appelgren LE, Ullberg S (1966) High resolution autoradiography of the adrenal medulla after injection of tritiated dihydroxyphenylalanine (Dopa). J Ultrastruct Res 14:277–293
Fillion G, Nosal R, Uvnas B (1971) The presence of a sulphomucopolysaccharide protein complex in adrenal medullary cell granules. Acta Physiol Scand 83:286–288
Geissler D, Martinek A, Margolis RU, Skrivanek JA, Ledeen R, Konig P, Winkler H (1977) Composition and biogenesis of complex carbohydrates of ox adrenal chromaffin granules. Neuroscience 2:685–693
Hartman BK, Udenfried S (1970) Immunofluorescent localization of dopamine-β-hydroxylase in tissues. Molec Pharmacol 6:85–94
Hempel K, Kraft M (1968) Zum Brenzkatechinamin-Stoffwechsel der Nebenniere. Licht- und elektronenmikroskopisch-autoradiographische Untersuchungen mit 3H-Dopa an Mäusen. Zell forsch 85:322–342
Hempel K, Männl FK (1967) Über die Bildung von H-3-Dopa aus H-3-Tyrosin und die Bestimmung der Dopa-Neubildungsrate in der Nebenniere des Huhnes und der Katze unter in vivo-Bedingungen. Naunyn-Schmiedebergs Arch Pharmacol 257:391–408
Hillarp NA (1959) Further observations on the state of the catecholamines stored in the adrenal medullary granules. Acta Physiol Scand 47:271–279
Kaufman S, Friedman S (1965) Dopamine-β-hydroxylase. Pharmacol Rev 17:1–100
Kent C, Williams MA (1974) The nature of hypothalamo-neurohypophyseal neurosecretion in the rat. A study by light and electron microscope radioautography. J Cell Biol 60:554–570
Kirshner N (1959) Biosynthesis of adrenaline and noradrenaline. Pharmacol Rev 11:350–357
Kirshner N, Goodall McC (1957) Formation of adrenaline from noradrenaline. Biochim Biophys Acta 24:658–659
Kirshner N, Kirshner AG (1971) Chromagranin A, dopamine β-hydroxylase and secretion from the adrenal medulla. Phil Trans R Soc Series B 261:279–289
Kobayashi S, Kent C, Coupland RE (1978) Observations on the localization of labelled amino acid in mouse adrenal chromaffin cells after the injection of L[4,5-3H] leucine. J Endocrinol 78:21–29
Margolis RU, Margolis RK (1973) Isolation of chondroitin sulfate and glycopeptides from chromaffin granules of adrenal medulla. Biochem Pharmacol 22:2195–2197
Margolis RK, Jaanus SD, Margolis RU (1973) Stimulation by acetylcholine of sulfated mucopolysac-charide release from the perfused cat adrenal gland. Molec Pharmacol 9:590–594
Morré DJ, Keenan TW, Huang CMP (1974) Membrane flow and differentiation: origin of Golgi apparatus membranes from endoplasmic reticulum. In: Ceccarelli B, Clementi F, Meldolesi J (eds) Advances in cytopharmacology, Vol 2. Raven Press, New York
Pletscher A, DaPrada M, Berneis KH, Steffen H, Luttold B, Weder HG (1974) Molecular organization of amine storage organelles of blood platelets and adrenal medulla. In: Ceccarelli B, Clementi F, Meldolesi J (eds) Advances in cytopharmacology, Vol 2. Raven Press, New York
Ravazzola M (1976) Intracellular localization of calcium in the chromaffin cells of the rat adrenal medulla. Endocrinology 98:950–953
Rosell S, Sedvall G, Ullberg S (1963) Distribution and fate of dihydroxyphenylalanine 2-14C (DOPA) in mice. Biochem Pharmacol 12:265–269
Smith AD (1968) Biochemistry of adrenal chromaffin granules. In: Campbell PN (ed) The interaction of drugs and subcellular components in animals. Churchill, London
Smith AD, Winkler H (1967) Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J 103:483–492
Williams MA (1969) The assessment of electron microscope autoradiographs. In: Barrer R, Costelt V (eds) Advances in optical and electron microscopy. Academic Press, New York
Williams MA (1973) Electron microscopic autoradiography: its application to protein biosynthesis. In: Campbell PN, Sargent JR (eds) Techniques in protein biosynthesis, Vol 3. Academic Press, NewYork
Winkler H, Hortnagl H, Schopf JAL, Hortnagl H, zur Nedden G (1971) Bovine adrenal medulla: synthesis and secretion of radioactively labeled catecholamines and chromogranins. Naunyn-Schmiedebergs Arch Pharmakol 271:193–203
Winkler H, Schopf JAL, Hortnagl H, Hortnagl H (1972) Bovine adrenal medulla: subcellular distribution of newly synthetized catecholamines, nucleotides and chromogranins. NaunynSchmiedebergs Arch Pharmakol 273:43–61
Yunghans WN, Keenan TW, Morré DJ (1970) Isolation of Golgi apparatus from rat liver. 3. Lipid and protein composition. Exp Molec Pathol 12:36–45
Author information
Authors and Affiliations
Additional information
Acknowledgements. This work was supported by a grant from the Medical Research Council of Canada to the Multidisciplinary Research Group of Hypertension of the Clinical Research Institute of Montreal and by the Canadian Heart Foundation
Rights and permissions
About this article
Cite this article
Benchimol, S., Cantin, M. Ultrastructural radioautography of synthesis and migration of proteins and catecholamines in the rat adrenal medulla. Cell Tissue Res. 225, 293–314 (1982). https://doi.org/10.1007/BF00214683
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214683